Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1403
Research Article(ISSN: 2644-1403) 
A Clinical Study to Assess the Effects of Pneumoperitoneum on Respiratory Mechanics and Hemodynamics in Laparoscopic Cholecystectomy in Obese Patients Volume 1 - Issue 1
Abhay Singh Bhadauria, Jitendra Agrawal*, Rakhi Mittal and Bhanu Choudhary
- Department of Anesthesiology, Osaka Medical College, Japan
Received: January 30, 2019; Published: February 21, 2019
Corresponding author: Jitendra Agrawal, Department of Anaesthesiology, Pain & Critical Care, GR Medical College, Gwalior (M.P) India
DOI: 10.32474/GJAPM.2019.01.000105
Abstract
Aim: To assess the effects of pneumoperitoneum on respiratory Mechanics and Hemodynamics in Laparoscopic Cholecystectomy
Background: Laparoscopic surgeries have been performed now a day’s very frequently. The pneumoperitoneum created in these laparoscopic procedures produces various effects on haemodynamic and respiratory parameters of the patients.
Methods: 61 patients of ASA grade I and II undergoing laparoscopic cholecystectomy were registered for this study. After induction of GA these patients were ventilated mechanically with tidal volume of 8ml/ kg and RR of 18/min. Haemodynamic (HR, SBP, DBP and MAP) and respiratory mechanics parameter (Peak inspiratory pressure, peak plateau pressure, et CO2, spO2 and end tidal volume) and arterial blood samples (pH, paCO2 and base deficit/excess) were recorded and analyzed at 30min intervals after creation of pneumoperitoneum with CO2. Changes in hemodynamic, respiratory mechanics and ABG were compared between the different time intervals with the base line values.
Results: a) Pneumoperitoneum insufflations produced significant increase of heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP).
b) It produced significant increase in peak inspiratory pressure (PIP), peak plateau pressure (PPP) and end tidal CO2 throughout the period of pneumoperitoneum and even after desufflation of pneumoperitoneum.
c) Arterial blood gas analysis during the period of pneumoperitoneum showed that PaCO2 remain increased even after 30min of desufflation of pneumoperitoneum in the recovery room and pH was lowest at 90min after insufflation and remain decreased after 30min of desufflation of pneumoperitoneum in the recovery room.
Keywords: Pneumoperitoneum; Haemodynamic; Arterial blood gas; Respiratory mechanics; Laparoscopy
Introduction
Obesity is an important respiratory risk factor after surgery. Morbid obesity has deleterious effects on respiratory mechanics, decreasing lung volumes, functional residual capacity, compliance and arterial oxygenation [1,2]. In a study by casati. it was shown that general anesthesia with reverse Trendelenburg position and pneumoperitoneum (PPM) improved lung compliance as well as gaseous exchange in morbidly obese patients undergoing surgery [3]. Laparoscopic surgical techniques seem beneficial in obese patients in terms of respiratory morbidity with a faster reachievement of normal respiratory function. Pneumoperitoneum plays a very important role in laparoscopic procedures; it provides working space to the surgeons for introduction of instruments intra abdominally. CO2 is the preferred gas for creation of pneumoperitoneum, but it is associated with the systemic absorption of CO2, which may have detrimental cardio respiratory effects. There is not much information about intraoperative respiratory mechanics related to pneumoperitoneum in the morbidly obese. As a result, there has been renewed interest in the anesthetic management of this group of patients. Respiratory mechanical properties before or after laparoscopic surgery have been studied [4]. However, there is little information in the literature about intraoperative changes in respiratory mechanics and patient tolerance to pneumoperitoneum (PPM). The aim of this study was to evaluate the effects of PPM on respiratory mechanics and blood gases in obese patients undergoing laparoscopic cholecystectomy.
Methods
After obtaining the approval from Institutional Ethics Committee, the study was carried out on 61 obese patients in the age group of 20-50 years of either sex belonging to ASA grade I or II, scheduled for elective laparoscopic cholecystectomy under general anesthesia. All patients undergo proper preanesthetic assessment and an informed written consent taken from all the patients. Patients were premedicated with Inj. fentanyl 1-2μg/kg BW followed by preoxygenation with 100% oxygen for 3 minutes by facemask. Induction of General anesthesia was done with in Thiopentone Sodium 5mg/kg BW. Endotracheal intubation was facilitated with intravenous Succinylcholine 1.5mg/kg BW and ventilation with 100% oxygen for 120 seconds. General anesthesia was maintained with nitrous oxide & oxygen (66:33), loading and maintenance dosage of inj Atracurium (0.1mg/kg) according to BW and halothane 0.5-1.0%. Lungs were mechanically ventilated with Mindray Wato Ex-20 Ventillator a volume- cycled ventilator using a closed circle system incorporating a carbon dioxide absorber. The ventilator was inspected for leak test before each case. Tidal volume is set at 8ml/kg, with a rate of 18 breaths/ min during surgery. Following variables were recorded i.e. peak inspiratory pressure, mean inspiratory pressure, expiratory tidal volume. The expired tidal PCO2, oxygen saturation PaO2, NIBP, HR, RR were monitored by Mindray Bene View T5 Moniter. During laparoscopy intraabdominal pressure was maintained automatically at 10mm Hg by a CO2 insufflator. Arterial blood sample was drawn from the radial artery with a 26G needle in a 2.5ml heparinized syringe, and analysis was done with a Eschweiler Combi line blood gas analyzer and variable i.e. PaCO2 and pH was recorded. Respiratory variables and hemodynamic parameters were recorded 15min after induction then 0, 30, 60 and 90min after creation of pneumoperitoneum and after desufflation (Ti, T0, T30, T60, T90, Td respectively). Arterial blood was sampled 10min after the induction and before pneumoperitoneum then 0 ,30, 60 and 90min after creation of pneumoperitoneum and after desufflation (Ti, T0, T30,T60, T90, Td respectively) and in the recovery room (30min after tracheal extubation) (Tr). Carbon dioxide was used for peritoneal insufflations, and abdominal pressure was maintained at 8-10mmHg. Throughout the procedure for any 20% rise in MAP above the basal MAP, halothane/ Inj. Dexmeditomidine/ or both was given to maintain the basal MAP and the case was excluded from the study group. For fall in MAP more than 20% of the basal MAP, halothane was decreased or stopped. Heart rate less than 50bpm was treated with Atropine 0.6mg intravenously. After surgery, patients were reversed with Inj. Glycopyrrolate 0.005mg/kg and Neostigmine 0.08mg/kg intravenously. After extubation patients were observed for recovery time defined as time to vocalize after extubation. The observations were recorded and tabulated. The statistical analysis was carried out by using statistical software SPSS 17 and paired student ‘t’ test for intra- group comparison was applied. P value of p>0.05 and p<0.05 were considered as statistically insignificant and significant respectively, and p-value <0.01 taken to be statistically highly significant.
Results
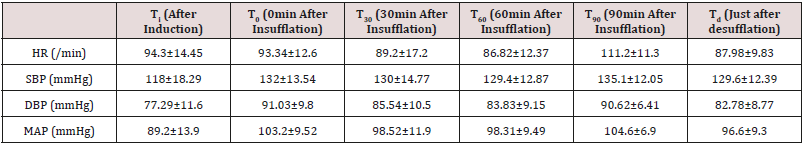
There was even distribution of age in study groups with majority of patients belonging to the age group of 20-60 years, majority of patients were females (M:F was 3:58), having mean weight (in kgs) of 56.38±4.71 and mean height 148.2±2.01 (in cms). The mean±SD of duration of anaesthesia (min) was 90.8±31.58 minutes and duration of pneumoperitoneum (min) was 56.6±23.3 minutes. As shown in (Table 1) the mean (±SD) of HR decreased insignificantly just after the creation of pneumoperitoneum, which then decreased progressively upto 60 minutes after pneumoperitoneum which was significant. The mean HR increased significantly 90 minutes after pneumoperitoneum but decreases significantly (p<0.05) after release of pneumoperitoneum. On comparing the baseline values of mean (±SD) systolic blood pressure, diastolic blood pressure and mean arterial pressure with values after induction of general anesthesia, there was significant (p<0.05) increase in SBP, DBP and MAP was observed immediately, 30min, 60min, 90min after insufflation and even after desufflation.
The mean±SD of peak inspiratory pressure (PIP) and peak plateau pressure (PPP) after induction was 14.5±2.4cm H2O and 6.61±1.0cm H2O respectively. When compared with baseline values significant (p<0.01) increase in PIP was seen at all time including after desufflation. The mean±SD of end tidal CO2 and expiratory tidal volume after induction was 29.0±4.1mmHg and 397.16±40.08ml respectively. On comparing with baseline values significant (p<0.01) increase in end tidal CO2 were observed immediately after insufflation, 30 min, 60min, 90min after insufflation and after desufflation but insignificant increase was observed in expiratory tidal volume. Significant rising trend in PaCO2 was observed throughout the period of pneumoperitoneum and remained increased even after desufflation of pneumoperitoneum. There was significant decreasing trend in pH observed immediately after insufflations, 30min, 60min, 90min after insufflation, after desufflation and 30min after extubation in the recovery room. It was lowest at 90min after insufflation and remained decreased after desufflation. The mean±SD of base excess after induction was -2.32±4.84mEq/l. As compared to baseline values there was insignificantly decrease in base deficit just after insufflation, thereafter base excess increases significantly at 30min and at 60min after insufflations, after desufflation and 30min after extubation in the recovery room, but it decreased insignificantly at 90min after insufflation of pneumoperitoneum.
Discussion
This study shows that there was decrease in HR initially which further decreased significantly to 60 minutes after pneumoperitoneum then increased significantly 90 minutes after release of pneumoperitoneum. This may be because of vagal stimulation due to peritoneal stretching during pneumoperitoneum insufflations later on it increases which may be due to central nervous system activation by CO2 accumulation and it evokes sympathoadrenal activation causing tachycardia but after release of pneumoperitoneum it decreased significantly [5,6]. The significant increase in mean MAP values were observed immediately after insufflations, 30min, 60min ,90min after insufflation and after desufflation. During whole pneumoperitoneum, significant changes in MAP were observed. These were due to multifactorial reason first by central nervous system mediated increased myocardial contractility, tachycardia and hypertension. Secondly, due to sympathoadrenal activation by increasing the systemic vascular resistance through the potential mediators i.e. catecholamines, prostaglandins, the rennin-angiotensin system and vasopressin [7-9]. There was significant increase in peak inspiratory pressure and peak plateau pressure was observed immediately after the insufflations, 30min, and 90min after insufflation and after desufflation [10,11]. The reason these changes during laparoscopic procedures may be respiratory embarrassment due to mechanical effect of increased intra-abdominal pressure by pneumoperitoneum. Abdominal distenstion markedly alters respiratory system mechanics, primarily by the abdominal expansion [12,13]. Changes in End tidal CO2 shows a significant increase immediately after insufflation 30min 60min, 90min after insufflations and after desufflation. The Increment of end tidal CO2 may attribute to increase absorption of CO2 into the systemic circulation through the peritoneal surface during pneumoperitoneum and hypoventilation due to elevated diaphragm, if ventilatory parameters were not adjusted accordingly it may lead to hypercabia and acidosis [14]. Significant rising trend in PaCO2 was observed immediately after insufflations 30min, 60min, 90min after insufflations, just after desufflation and 30min after extubation in the recovery room. The pathophysiology of these effects is mainly due to the consistent increase in paCO2 because of CO2 absorption from peritoneal cavity and hypoventilation due to elevated diaphragm. There was significant decreasing trend in pH observed immediately after insufflations 30min, 60min, 90min after insufflations and 30min after extubation in the recovery room after desufflation. The consistent decreasing trend in pH was due to significant absorption of CO2 from the peritoneal cavity and increased metabolic CO2 production after the release of pneumoperitoneum [15]. There was insignificantly decrease in base deficit just after insufflation thereafter base excess increased significantly at 30min and at 60min after insufflations after desufflation and 30min after extubation in the recovery room, but it decreased insignificantly at 90min (0.27±2.82mEq/l) after insufflation of pneumoperitoneum [16,17].
Conclusion
Pneumoperitoneum insufflations with CO2 in laparoscopic cholecystectomy in obese patients produces significant consequences including increase of heart rate, systolic blood pressure, diastolic blood pressure and mean arterial pressure, peak inspiratory pressure, peak plateau pressure and end tidal CO2. There was increase in PaCO2, decrease in pH but Base excess increased significantly. These changes are due to absorption of CO2 via the pneumoperitoneum, vagal stimulation, pressure due to pneumoperitoneum. The anesthesiologists should carefully observe and document respiratory mechanics and arterial blood gases in these patients.
References
- Porhomayon J, Papadakos P, Singh A, Nader ND (2011) Alteration in respiratory physiology in obesity for anesthesia-critical care physician HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 3(2): 109-118.
- Sprung J, Whalley DG, Falcone T, Warner DO, Hubmayr RD, et al. (2002) The impact of morbid obesity, pneumoperitoneum, and posture on respiratory system mechanics and oxygenation during laparoscopy. Anesth Analg 94: 1345-1350.
- Casati A, Comotti L, Tommasino C, Leggieri C, Bignami E, et al. (2000) Effects of pneumoperitoneum and reverse Trendelenburg position on cardio-pulmonary function in morbidly obese patients receiving laparoscopic gastric banding. Eur J Anaesthesiol 17: 300-305.
- Perilli V, Sollazzi L, Bozza P, Modesti C, Chierichini A et al. (2000) The effects of reverse Trendelenburg position on respiratory mechanics and blood gases in morbidly obese patients during bariatric surgery. Anesth Analg 91: 1520-1533.
- Jung KT, Kim SH, Kim JW, So KY (2013) Bradycardia during laparoscopic surgery due to high flow rate of CO2 insufflation. Korean J Anesthesiol 65(3): 276-277.
- Koivusalo AM, Kellokumpu I, Scheinin M, Tikkanen I, Makisalo (1998) Comparison of gasless mechanical and conventional carbon dioxide pneumoperitoneum methods for laparoscopic cholecystectomy. Anesth Analg 86(1): 153-158.
- Androne I, Tsounaki E, Katergianakis V, Manouras A, Bramis I (2012) Arterial blood gas and haemodynamic changes due to pneumoperitoneum in laparoscopic cholecystectomy. Hellenic Journal of Surgery 84(4): 236-242.
- Lindgren L, Koivusalo AM, Kellokumpu I (1995) Conventional pneumoperitoneum compared with abdominal wall lift for laparoscopic cholecystectomy. Brit J Anaesth 75(5): 567-572.
- Umar A, Mehta KS, Mehta N (2012) Evaluation of hemodynamic changes using different intra-abdominal pressures for laparoscopic cholecystectomy. Indian J Surg 75(4): 284-289.
- Bardoczky GI, Engelman E, Levarlet M, Simson P (1993) Ventilatory effects of pneumoperitoneum monitored with continuous spirometry. Anaesthesia 48(4): 309-311.
- Hirvonen EA, Nuutinen LS, Kauk M (1995) Ventilatory effects, blood gas changes, and oxygen consumption during laparoscopic hysterectomy. Anesth Analg 80(5): 961-966.
- Iwasaka H, Miyakawa H, Yamamoto H, Kitano T, Taniguchi K (1996) Respiratory mechanics and arterial blood gases during and after laparoscopic cholecystectomy. Can J anaesthesia 43(2): 129-133.
- Maharjan SK, Shrestha BR (2007) Do we have to hyperventilate during laparoscopic surgery? Kathmandu Univ Med J (KUMJ 19: 307-311.
- Serpa Neto A, Filho RR, Cherpanath T, Paulus F, Tuinman PR, et al. (2016) Associations between positive end-expiratory pressure and outcome of patients without ARDS at onset of ventilation: a systematic review and meta-analysis of randomized controlled trials. Ann Intensive Care 6(1): 109.
- Shobhana G, Gadani H, Mita P (2009) Comparative Clinical Study of Preinsufflation Versus Postdesufflation Arterial Blood Gas Analysis in Laproscopic Surgeries. The Internet Journal of Anesthesiology 25(1).
- Chang YJ, Myung HY, Jae HN (1995) Changes in Arterial Blood Gases during Laparoscopic Cholecystectomy under the General Anesthesia. Korean J Anesthesiol 28(5): 682.
- Kwak HJ, Jo YY, Lee KC, Kim YB, Shinn HK, Kim JY (2010) Acid-base alterations during laparoscopic abdominal surgery: a comparison with laparotomy. Br J Anaesthesia 105(4): 442-447.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...