
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4676
Research Article(ISSN: 2637-4676) 
Field Treatment Effects on Seed Germination and Early Growth Traits of Berseem Clover under Salinity Stress Conditions Volume 2 - Issue 1
F Daneshnia1 and MR Chaichi2*
- 1Department of Agronomy and Plant Breeding, University of Tehran, Iran
- 2Plant Science Department, Huntley College of Agriculture, California State Polytechnic University, Pomona, USA
Received: March 06,2018; Published: April 11,2018
Corresponding author: MR Chaichi, Plant Science Department, Huntley College of Agriculture, California State Polytechnic University, Pomona, USA
DOI: 10.32474/CIACR.2018.02.000127
Abstract
Developing seeds with a high level of tolerance against salinity and water shortage scan guarantee best seedling establishment in arid and semi-arid agricultural lands. This three-year field, laboratory and greenhouse study proposes the surfactant application to effectively prevent the detrimental impacts of severe environmental conditions on the development of parental plants. The germination rate of six parental seeds of berseem clovers (Trifolium alexandrinum L.) from I100 (100% irrigation water)/I100+s (irrigation water with surfactant, s), I75/I75+s and I50/I50+s treatments are studied under seven osmotic salinity potentials (0, -0.2, -0.4, -0.6, -0.8, -1, and -1.2MPa). Plants from seeds developed by surfactant in full and moderate limited irrigation (I100 and I75) show a favorable germination percentage when under saline stress treatments of -0.4 and -0.8MPa. The highest seedling lengths of 10.2 and 10.3cm were achieved for the seeds produced from I75 and I100+s treatments, respectively. Utilizing the surfactant across all field treatments had a positive effect on the weighted germination index compared to the counterpart treatment. In addition, Seedlings from seeds treated by surfactant in the germination tests have higher shoot/root ratios, which show the efficiency of surfactant application in promoting a better root and shoot development under saline/drought stresses.
Core ideas
a) Surfactant application enhanced parental seeds tolerance against salinity conditions.
b) Seeds developed by severely limited irrigation (I50) had a higher G% (75%).
c) Highest seedling lengths (10.2cm) obtained from plants treated by I75+ surfactant treatment.
d) Shoot/root ratio enhanced by surfactant application under salinity stress.
Abbrevations: G%: Germination Percentage; WGI: Weighted Germination Index; PPM: Mg per Liter; PPFD: Photosynthetic Photon Flux Density; BNS: Basal Nutrient Solution; AT Pase: Adenosinetri Phosphates; MGT: Mean Germination Time
Introduction
Seed performance refers to a capacity of seeds to germinate under various environmental conditions and relates to a critical period of a plant's life cycle which is eminently important in ecological and agronomic plant growth [1]. There is a general agreement that the performance and viability of seeds during their early stages of germination may be related to the conditions under which seeds are formed, developed, and matured [2]. As seed germination is the beginning of a plant's life cycle, proper seedling emergence is critically important for a decent establishment of plant population [3]. Many researchers have reported that adverse environmental conditions affect crop growth and productivity, postponing the seed germination and reducing the germination rate [4-7]. Plants experience a variety of abiotic stresses such as high/low temperatures, drought and salinity stress during their lifecycles which have a profound effect on their viability, growth, morphology, and reproduction [8]. In arid and semi-arid regions, seed germination and emergence are considered as critical stages for plant establishment and crop growth, which can elevate the crops' density and yields Shaygan 2017. In these regions, soil salinization is detrimental to plant growth and productivity [9,10].
Plants usually take up salt (Na+ and Cl- ions) from water which is detrimental to germination [11-13]. Plant growth in saline dry and semi-dry soils can be further damaged by water scarcity due to high osmotic pressure, which prevents adequate water uptake [14]. (Tables 1 & 2) present the summary of recent reports onthe adverse effects of salt/drought stress on seed germination and establishment [15-18]. For instance, the seed germination, emergence rate and growth of young seedlings of sugar beet (Beta vulgaris L.) [19,20], Phaseolus mungo [21] and Dianthus chinensis L. [22] decreased by NaCl treatments. Quantitatively, the salinity condition decreased the seed germination of nut (atropha curcas L.) by 50% [23], coriander (Coriandrum Sativum L.) by 65.67%, cultivar Kalmiand Pant Haritma by 55.56% [24], oat (Avena sativa L.) by 60.87% [25] etc. Berseem clover (Trifolium alexandrinum L.) is one of the best forage sources for feeding live stock and is considered as anon-bloating and high-quality forage [26].
Table 1: Recent studies on the effects of salinity/drought stresseson qualitative and quantitative traits of plants.
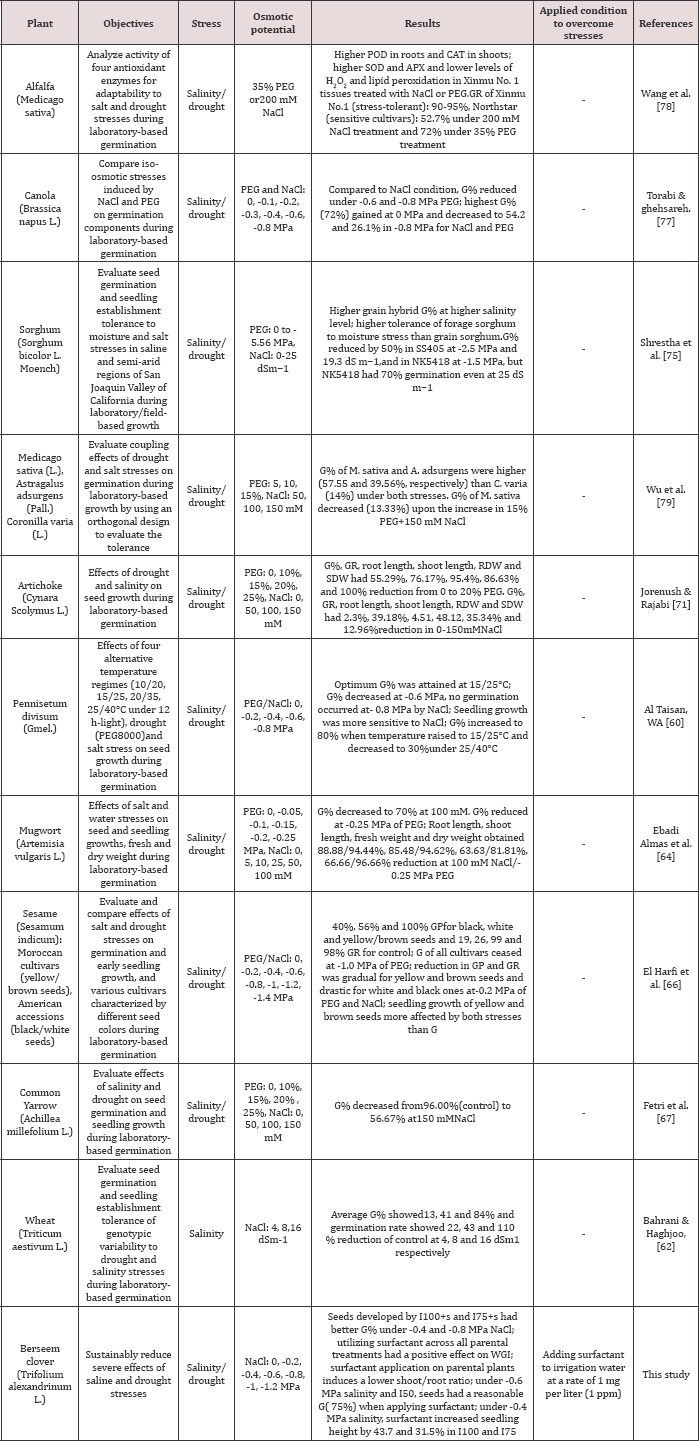
G%= Germination percentage, GR=Germination Rate, SDW=Seedling Dry Weight,RDW=Root Dry Weight, SOD=Superoxide of dismutase, CAT=Chloramphenicol Acetyltransferase, APX=Ascorbate Peroxidase, PEG=Polyethylene Glycol
It is moderately tolerant to salinity compared to wheat and crops [28-30] (Tables 1 & 2). Thus, there is limited information strawberry clover [27]. Despite the advantages, studies on the on the effect of field treatments (seed production conditions) effects of salt stress have been mostly carried out on agricultural on subsequent salt stress tolerance at the germination stage and seedling establishment of legume forage crops such as berseem clover. Water shortage and saline soil are known as one of the main natural hazards especially in arid and semiarid regions which delay seed germination and seedling establishment [31]. The aims of this three-year study are to determine factors responsible for the failure of germination and early growth characteristics of berseem clover seeds under saline/osmotic conditions. (Tables 1 & 2) present the results of recent studies on the reduction effect of salinity and drought conditions on different plants traits (Tables 1 & 2). Compared to these results, the present study proposesane co-friendly surfactant solution to mix with irrigation water at a very low rate of 1mg per liter (1ppm) to substantially reduce the severe effects of salinity and drought stresses on seed germination and growth of berseem clover.
Table 2: Recent studies on the effects of salinity/drought stresses on qualitative and quantitative traits of plants.
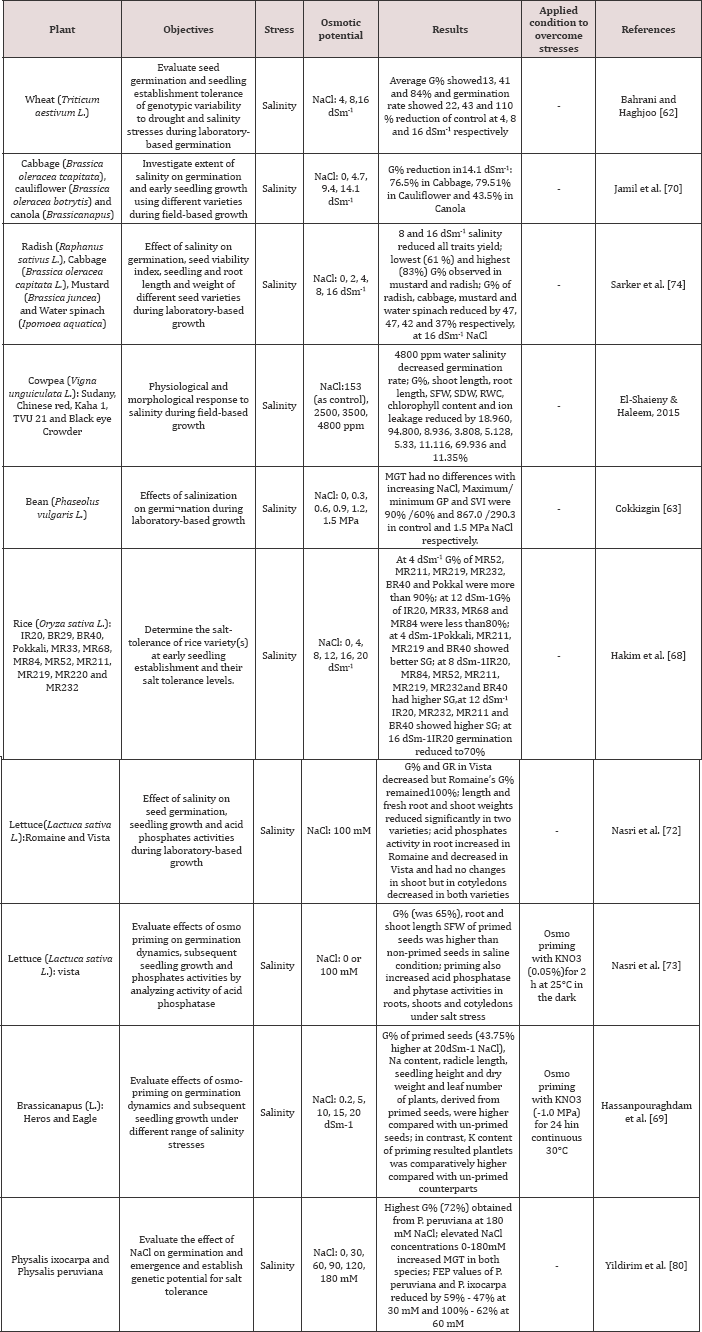
G%=Germination percentage, GR=Germination Rate, MGT=Mean Germination Time, FEP=Final Emergence Percentage, SVI=Seed Vigor Index, SFW=Seedling Fresh Weight, SDW=Seedling Dry Weight, RWC=Relative Water Content, SG=Speed of Germination.
Materials and Methods
Seed materials
Berseem clover (Trifolium alexandrinum L.) seeds obtained from our field experiments during the 2013-2015 growing seasons at the Research Farm of the College of Agriculture, University of Tehran, Karaj, Iran (N 35°56', E 50°58’) [32] Daneshnia 2016. The climate at this site is considered as arid to semiarid with a longterm (50years) mean air temperature of 13.5°C, soil temperature of 14.5°C, and 262mm of mean annual rainfall. In the field experiments, three levels of irrigation were applied to the main plots, including normal irrigation I100 (replenishment of 100% of weekly evaporation and plant water requirement), moderate irrigation (I75) and limited irrigation (I50). Two types of water treatment, including tap water and water+surfactant (added at a rate of 1ppm), were applied to the subplots. The experiments were carried out in split-split plots based on a completely randomized block design with three replications. Likewise, the irrigation treatments (I100, I75, and I50) were scheduled based on the common practice of the area, once a week, when the plants reached the 4 to 6-leaf growth stage. The amount of required irrigation water was calculated using the following equation [26,32].
In = 0.623 x A x Kc x ET0 x IE-l (1)
Where In is the volume of irrigation water (gallons), 0.623 is a constant, A is the canopy area (sq.ft.), Kc is the crop coefficient, ET0 is the weekly potential evapo transpiration (inches), and IE is the irrigation efficiency.
Salt stress germination test
The effects of six parental treatments (three irrigation levels and two water treatments) under salinity conditions were systematically studied during the germination process. These experiments were performed at the Seed Research Laboratory of the College of Agriculture, University of Tehran. The salinity treatment was built with seven osmotic potentials of 0, -0.2, -0.4, -0.6, -0.8, -land -1.2 MPa by, respectively, using 0, 2.66, 5.3, 7.99, 10.65, 13.31 and 15.98 gr/kg NaCl in dematerialized water. 50 seeds from each parental treatment were placed in 9cm sterile Petri dishes which contained two what man No.1 filter papers, and then placed in seed germination at 20oC. 10ml solution from each test was poured onto the plate; the papers were altered once after every 2 days to prevent salt accumulation [33]. The 42 treatments (6 parental treatments and 7 salinity levels) were arranged on a factorial base with a completely randomized design and three replications. To maintain water and salt concentrations near the target levels, required dematerialized or saline water was added to the Petri dishes during the daily monitoring. The number of germinated seeds was recorded every 24 hours for two weeks. A seed was considered as germinated when an emerging radical was longer than 2mm. Then, the germination parameters, such as final germination percentage (G%) and weighted germination index (WGI) [34], were determined with higher weights assigned to the seeds that germinated early and less to those that germinated late. The final WGI values (after 14 days) were calculated using the method proposed by Bu [34]
WGI = {N x n1 + (N - l)x n2 +---+lx n14}/N x N' (1)
Here, n1, n2, ...., n10 is the number of seeds that germinated on the 1st, 2nd and subsequent days until the 10th day, respectively, N is the total days of germination process, and N' is the total number of seeds placed in incubation. In Eq.1, the final WGI value is the product of the experimental value of WGI and the percentage of a live seeds in each treatment.
Early growth characterization of berseem clover seedlings under saline condition
Following the salt stress germination test, a series of pot experiments were conducted to evaluate the interaction between field treatment, salinity, and surfactant in the early growth of berseem clover seedlings. These experiments were performed at the Research Greenhouse of the College of Agriculture, University of Tehran. The greenhouse temperature, humidity and photosynthetic photon flux density (PPFD) for day/night were 25/20°C, 65/60%, and 300-600 μmol m-2s-1, respectively. The salinity treatments comprised of either intact soil from the field (soil with an electrical conductivity (EC) of 0.075 MPa and a primary osmotic potential of (-0.083MPa) or saline soil with an osmotic potential of -0.4MPa. The osmotic potential of salinity stress (-0.4MPa) was chosen on the basis of the optimum results of osmotic potential treatments from saline stress in the germination tests. In the following, the salinity level of the pots was kept at -0.4MPa throughout the experimental period. After a few preliminary tests, the EC of soil was adjusted to -0.4MPa by adding a solution of 5.3g NaCl+100 ml distilled water to all the pots which contained the saline-treated soil. All the pots were filled with 2.43kg of air-dried soil collected from the field. The soil of the pots was sieved (with a mesh diameter of 1 cm) and loaded in plastic pots 15cm in diameter and 12cm in height.
The field soil texture was clay loam with the following physicochemical characteristics: pH=7.8, EC=2.31 dSm-1, total nitrogen (N)=0.09%, available phosphorus (P)=8.87 mgkg-1, and available potassium (K)=225mgkg-1. The soil in each pot was fertilized with 1 liter of basal nutrient solution (BNS) which mainly consisted of both ammoniums (NH4) and nitrate (NO3) as the source of nitrogen [35]. To study the effect of surfactant on seedling establishment of parental seeds in saline environments, the pots were sprinkled with different levels of fresh water with/without surfactant additive. The soil was watered once every three days to replenish 100% of the soil moisture to ensure the plants were under no drought stress. The seeds used in this experiment were the same as used in the germination test. The nonionic surfactant marketed with the name Golden Irrig. Aid was provided by Aquatrols Corporations, New Jersey, USA (10% Alkoxylatedpolyols, 7% Glucoethers, Inter ingredient 83% water). The surfactant was applied at a rate of 1mg per liter (1ppm) to the irrigation water for water treatment purposes [36,37].
Statistical Analysis
The ANOVA technique with three replications was applied to the randomized design using the Proc GLM procedure SAS [38]. Mean comparison was implemented using Duncan's test at the 95% level of probability.
Results
Salt stress germination test
The laboratory experimental results showed that the germination is completely suppressed by -1 and -1.2 MP a salinity stress in the presence of the highest concentrations of NaCl. As the salinity increases, the germination percentage followed a decreasing trend across all field treatments. However, berseem clover can tolerate NaCl up to -0.4 MPa saline stresses with a moderate decrease in its germination stage (Figure 1). As the salinity stress increased from -0.4 to-0.6MPa, G(%) diminished significantly in all seed sources; the least (8.3%) and highest (33.3%) reductions were observed in I (with and without surfactant) and full irrigation (I100) (with and without surfactant)treatments, respectively. It is not able that under a higher salinity stress (-0.8 MPa), germination percentage increased across all parental seeds compared with -0.6MP a salinity stress. Parental seeds, collected from limited irrigation treatments ( I75 and I50) under various water treatments (with/without surfactant application), were influenced by the salinity level (Figure 1). Results indicated that the seeds, developed under drought stress during grain filling, were more tolerant to osmotic stress especially when the surfactant was added to the system. Under high salinity stress (-0.6MPa), seeds developed by severely limited irrigation (I50) have more tolerance to the osmotic pressure.
Figure 1: Parental effect (water stress along with surfactant application: I100/I100+s I75/I75+s, and I50/I50+s) on berseem clover seed germination under different saline conditions.
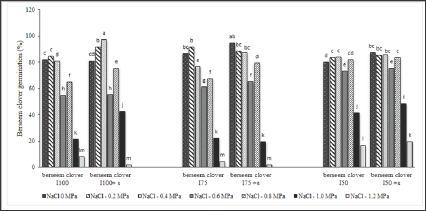
Figure 2: Parental effect (water stress and surfactant application) on berseem clover seed germination undermoderate and severe saline stress (-0.4 and -0.8MPa). (a) I100/-0.4MPa saline stress, (b) I100+s/-0.4MPa saline stress, (c) I75/-O.8 MPa saline stress, (d) I75+s/-0.8 MPa saline stress.
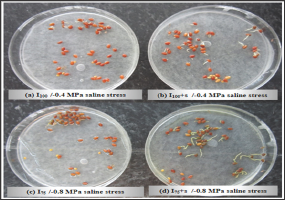
Figure 3: Parental effect (water stress along with surfactant application): I100/I100+s, I75/I75+s, and I50/I50+s) on berseem clover seed WGI under different saline conditions.

This showed a reasonable germination percentage (75%) when the utilized surfactant was added to the system. As the level of salinity stress increased, the application of surfactant led to a better performance in the preservation of the seeds germination under harsh conditions. As shown in Figure 2 (a-d), seeds developed with irrigation water plus surfactant in both full and moderately limited irrigation treatments (I100 and I75) have better germination rates, compared with their counterpart treatments with no added surfactant under saline stress (-0.4 and -0.8MPa). In all parental treatments, as salinity stress intensity increased from 0 to -1.2MPa, WGI followed a decreasing trend (Figure 3). The highest WGI (87) was gained from IWo+s under a moderate salinity stress (-0.4MPa). Under higher osmotic stresses, seeds produced by surfactant had a higher WGI across all parental treatments compared to their counter parts from non-surfactant treatments. This is the indication of an enhanced germination rate in parental seeds upon the application of surfactant in irrigation water. The WGI in moderate water potentials of -0.2 and -0.4 MPa, induced by NaCl, was higher compared to that under more stressed levels (Figure 3).
Parental effects on berseem clover seedling establishment and growth under saline condition
Table 3 indicates that the seeds produced under deficit irrigation regimes (75% and 50%) with surfactant application (parental treatments) have better tolerance to salinity stress. This is also seen in Figure 4 where seeds, collected from plants grown with the irrigation treatments of I75 and I50, have a better performance under salinity stress. The highest seedling height (10.2cm) is gained from I75+s where the pots are water reducing surfactant. Figure 5 presents the evidence where the application of surfactant significantly increased the berseem clover seedling height when subjected to saline stress treatments, compared to the control. Under saline conditions, surfactant application increased the seedling height by 43.7 and 31.5% in I100 and I75, respectively, compared to control. In fact, after the application of a surfactant, berseem clover height significantly increased across all treatments. Figure 6 shows that the seeds developed under moderate water stress had a better seedling growth and development with surfactant application ( I75+s), compared to the control.
Figure 4: Parental effect (water stress along with water treatment: I100, I75, and I50 ) and surfactant application on berseem clover seedling height under the saline condition of -0.4MPa in comparison with the normal condition.

Figure 5: Parental effect (water stress along with water treatment: I100+s, I75+s, and I50+s) and surfactant application on berseem clover seedling height under the saline condition of - 0.4MPa in comparison to normal condition.

Table 3:
Mean comparison was implemented using Duncan ' test at the 95% level of probability. Different letter's at each column indicate significant difference at p≤0.05, 0.01. *, ** show the significance at p≤0.05, 0.01, respectively.
Figure 6: Berseem clover (I75+s) seedling growth under the saline stress of -0.4MPa: (a) irrigation with asurfactant, (b) irrigation without surfactant.

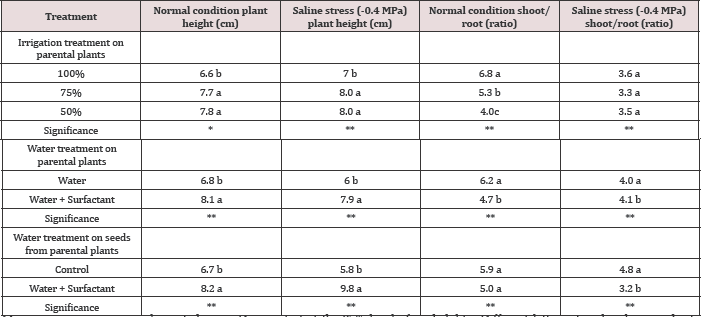
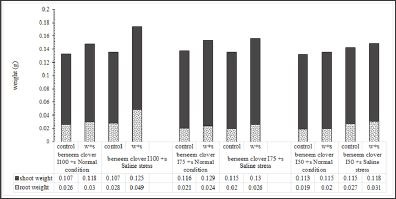
Seedlings from seeds developed under limited irrigation treatments (I50 and I75), had lower shoot/root ratios compared to those developed in I100 treatment (Figure 7). This phenomenon verifies the higher vegetative growth potential of seeds developed under full irrigation treatment (I100) (Figure 8). It is assumed that when the soil water content is inadequate to facilitate nutrient uptake by roots, plants face difficulties in absorbing the nutrients necessary for their growth. The higher vegetative growth potential of seeds is confirmed in (Figure 8) where the developed seeds have a lower root weight under a full irrigation treatment (I100). As shown in Figure 8, when the pots were watered with a surfactant, the plants’ shoot/root ratio significantly reduced by the favorable effects of surfactant on increasing the root growth under different growing conditions. Figure 9 clearly shows that seeds, developed under deficit irrigations ( I75 and I50 with surfactant), produced plants with higher shoot/root ratios compared to fully irrigated seeds. Using surfactant during the grain filling in parental plants induced a higher shoot/root ratio in developed seedlings, compared to their counterpart treatments. Figures 7 & 9 give clear evidence for this phenomenon which can be explained by the promoting effects of surfactant on better root development in the experiments.
Figure 7: Parental effect (water stress along with water treatment: I100, I75 ,and I50) and surfactant application on berseem clover's shoot/root ratio under the saline condition of -0.4MPa in comparison to normal condition.
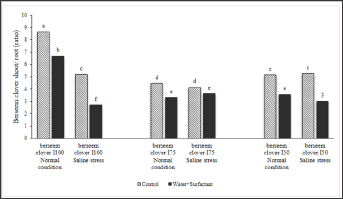
Figure 8: Parental effect (water stress along with water treatment: I100, I75, and I50) and surfactant application on berseem clover's seedling biomass (shoot and root) under saline condition of -0.4MPa) in comparison to normal condition.
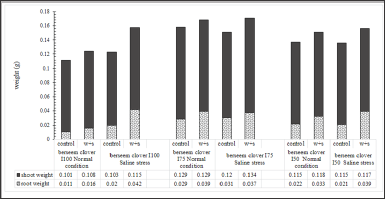
Figure 9: Parental effect (water stress along with water treatment: I100+s, I75+s, and I50+s) on berseem clover's shoot/root ratio under the saline condition of -0.4MPa in comparison to normal condition.
Discussion
It was previously reported that the application of surfactant had a modifying effect against the adverse condition of severely limited irrigation (I50) compared to full irrigation (I100); this resulted in maintaining the forage quality and quantity [26,32]. In this study, we conducted systematic experiments to specify the response of parental seeds (from deficit irrigation field treatments) to salinity stress.
Increased Salinity Tolerance of Seeds Under Field Treatments
Drought and salinity create multifaceted stresses that cause a significant reduction in crops yield depending on the plant growth stage, stress duration and severity Muscolo 2014. Germination is the most critical and sensitive stage in the life cycles of plants [38,39]. Seeds, exposed to unfavorable environmental conditions such as salinity and drought, may exhibit a change in the subsequent seedling establishment processes [40,41]. Our results highlighted the effects of field treatments (drought stress and surfactant application) on increasing seed germination under salinity stress. This study demonstrated that the seeds, developed under drought stress during grain filling, are more tolerant to osmotic stress which agrees with the report from Maleki [42]. The inhibitory effect of NaCl is because of the osmotic stress imposed on the seed/plant during the early phases of germination. At the early stages of imbibitions, the seed respiration and metabolic processes are highly correlated with the water uptake [43]. A threshold level of hydration is required in the initial steps of germination and their subsequent radical elongation [44]. By ensuring turgor maintenance, 'osmotic adjustment' may reduce sensitivity to water stress or allow the plant growth to continue even at a slow rate under stress conditions [45].
Salinity can affect the germination of seeds by disruption to the structure of enzymes and other macromolecules, damaging the cell organelles and the plasma membrane, disrupting the respiration, photosynthesis, and protein synthesis [46-48]. In the salinity stress of -0.6 to -1.2 MPa, a sharp reduction of WGI was observed across all parental seed resources. These results corresponded with the findings of Cavallaroa [49], who asserted that, due to osmotic pressure, the seed imbibitions becomes slower; thus, seed metabolism activation is postponed, and germination is taken place with a delay [50]. The amount of WGI resulted from the osmotic pressure of -0.2 and -0.4MPa, induced by NaCl, is higher compared to that under more stress levels. This phenomenon can be the result of increases in the expression of aquaporin and Adenosinetri phosphates (ATPase), as well as a rise in protease and lipase activities [51,52].
Improved seedling growth under parental treatments
Plants grown in the field under I75+ surfactant application had the highest seedling height (10.2cm). As explained by Maleki Farahani 2010, seeds germinate faster under severe water stress and have a shorter mean germination time (MGT), compared with the seeds produced under full irrigation. Probably, the increased physiological activity, due to the greater absorption of moisture by the treated seeds, is responsible for the improvement of germination and seedling. These results support the idea that the surfactant application in irrigating water in dry regions with salinity problems can maintain agricultural sustainability. The surfactant has a constructive modifying effect on the adverse effects of salinity stress compared with control (Table 3). According to a report from Demie [53], the positive effects of surfactant on improving the water movement and storage in soil could explain the results of the present study in the field. As such, surfactants have the positive effect of increasing the uniformity of water content in soil under drought conditions [54].
Researchers have reported that in response to soil salinity, the seedling growth, leaf area, root and shoot biomass are all reduced [55]. However, in the present study, the irrigation with surfactant successfully maintained a higher resistance of the seeds to saline stress conditions throughout the course of their growth and development (Table 3). As presented in Figure 5, the application of surfactant increased the berseem clover seedling height significantly while subjected to saline stress treatments, compared to the control. When the soil wet ability is less than the water requirement of plants, the use of surfactant in combination with appropriate irrigation and soil cultivation practices improves the soil hydrological behavior; this also improves the irrigation efficiency and water conservation [56,57]. Reported that salinity reduced water potential in the leaves of clovers (Trifolium sp.); shortened the length and lowered the dry mass of the stem, and undesirably affected the length and conductivity of plant roots. However, in this study, when the pots were treated with a surfactant, the plants' shoot/root ratio significantly reduced under all growing conditions. This result can be explained by the favorable effects of surfactant on increasing the root growth in plants (Figure 8).
Poulter [58] stated that the surfactants decrease the surface tension of water and, in this way, the penetration of water into the soil profile becomes easier, thus, the wetting area of soil increases. With the addition of a surfactant, the plant roots can find water in a wider soil profile, which leads to a better vegetative and generative growth and a higher efficiency in water usage. Figures 7 & 9 are a witness for promoting effects of surfactant on better root development in this experiment [59]. These results clearly show the efficiency of surfactant in increasing both the aboveground and underground components of plants in semi-arid regions where water is limited [60-64]. Our data indicate that those plants irrigated by surfactant and produced from seeds developed with surfactant have a higher shoot/root ratio under saline stress condition. The authors are currently carrying out physiological studies to better understand the function of surfactants in plants and its role in seed germination by tracking the absorption of surfactant into plants and seeds (Figure 10). This investigation is necessary to determine the contribution of surfactants to the metabolism of plants and seeds and other internal changes which lead to a better performance under saline stress conditions [65-71].
Figure 10: Parental effect (water stress along with water treatment: I100+s, I75+s, and I50+s) on berseem clover's seedling biomass (shoot and root) under the saline condition of -0.4MPa in comparison to normal condition.
Conclusion
Improving the seed tolerance to saline soil or water shortage can enhance forage production and agricultural sustain ability in arid and semi-arid areas. In this study, the application of surfactant increased the tolerance of berseem clover seeds to salinity stress under full, I , medium, I , and severe, I , irrigation treatments. Parental seeds developed under the severely limited irrigation in the field had a higher germination percentage (75%) compared to normal and moderately irrigated seeds [72,73]. Enhanced WGI of seeds, produced by surfactant across all parental treatments, indicated the desired effect of surfactant application. Plants treated by surfactant in irrigation water had a higher shoot/root ratio under saline stress condition. The results clearly showed the enhancement effect of surfactant application on improving seed performance and plant components in semi-arid regions where limited irrigation water and salinity are the main issues [74-81].
Acknowledgement
The financial support of the University of Tehran is gratefully acknowledged.
References
- Hanzi He, Deborah de SV, Basten Snoek L, Schnabel S, Nijveen H, et al. (2014) Interaction between parental environment and genotype affects plant and seed performance in Arabidopsis. Journal of Experimental Botany. 65(22): 6603-6615.
- Ansari O, Azadi MS, Sharif Zadeh F, Younesi E (2013) Effect of hormone priming on germination characteristics and enzyme activity of mountain rye (Secale montanum) seeds under drought stress conditions. Journal of Stress Physiology and Biochemistry 9(3): 61-71.
- Khan MA, Gulzar S (2003) Germination Responses of Sporobolus Ioclados: A Saline Desert Grass. Journal of Arid Environments 53: 387394.
- Patade VY, Maya K, Zakwan A (2011) Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Research Journal of Seed Science 4(3): 125-36.
- Rouhi HR, Aboutalebian MA, Sharifzadeh F (2011) Effects of hydro and osmopriming on drought stress tolerance during germination in four grass species. International Journal of Agricultural Sciences 1 (2): 107114.
- Ansari O, Choghazardi HR, Sharif Zadeh F, Nazarli H (2012) Seed reserve utilization and seedling growth of treated seeds of Mountain Ray (Secalemontanum) as affected by drought stress. Agricultural research in Moldova 2(150): 43-48.
- Ansari O, Sharif Zadeh F (2012) Osmo and hydro priming improvement germination characteristics and enzyme activity of Mountain Rye (Secale montanum) seeds under drought stress. Journal of Stress Physiology and Biochemistry 8(4): 253- 261.
- Deborah V, Malone RP, Dix PJ (2011) Increased tolerance to abiotic stress in tobacco plants expressing a barley cell wall peroxidase. Journal of Plant Sciences 6(1): 1-13.
- Osakabe Y, Kajita S, Osakabe K (2011) Genetic engineering of woody plants: current and future targets in a stressful environment. Physiologia Plantarum. 142(2): 105-117.
- Jamil M, W Iqbal, A Bangash, S Rehman, QM Imran, ES Rha (2010) Constitutive expression of OSC3H33, OSC3H50 and OSC3H37 genes in rice under salt stress. Pakistan Journal of Botany 42: 4003-4009.
- Khajeh Hosseini M, Powell AA, Bingham IJ (2003) The interaction between salinity stress and seed vigour during germination of soybean seeds. Seed Science and Technology 31(3): 715-725.
- Atak M, Kaya MD, Kaya G, Cikili Y, Ciftci CY (2006) Effects of NaCl on the germination, seedling growth and water uptake of triticale. Turkish Journal of Agriculture and Forestry 30: 39-47.
- Kaya MD, Okcu G, Atak M, Cikili Y, Kolsarici O (2006) Seed treatments to overcome salt and drought stress during germination in sunflower (HelianthusannuusL.). European Journal of Agronomy 24(4): 291-295.
- Sharifi Rad M, Sharifi Rad J (2013) Effects of Abiotic Stress Conditions on Seed Germination and Seedling Growth of Medical Plant, Hyssop (Hyssopusofficinalis L.). International Journal of Agriculture and Crop Sciences 5(21): 2593-2597.
- Erdal S, Aydin M, Genisel M, Taspinar MS, Dumlupinar R., et al. (2011) Effects of salicylic acid on wheat salt sensitivity. African Journal of Biotechnology 10(30): 5713-5718.
- Fethi B, Neila R., Mourad S, Nouari M, Mohamed EG (2011) Genetic adaptability of durum wheat to salinity level at germination stage. African Journal of Biotechnology 10(21): 4400-4404.
- Abbasi FM, Ahmad H, Perveen F, Inamullah Sajid M, Brar DS (2010) Assessment of the genomic relationship between Oryza sativa and Oryzaaustraliensis. African Journal of Biotechnology 9(12): 1312-1316.
- Patane C, Saita A, Sortino O (2012) Comparative Effects of Salt and Water Stress on Seed Germination and Early Embryo Growth in Two Cultivars of Sweet Sorghum. Journal of agro crop science 199(1): 30-37.
- Wang K, Liu Y, Dong K, Dong J, Kang J, et al. (2011) The effect of NaCl on proline metabolism in Saussurea amara seedlings. African Journal of Biotechnology 10(15): 2886-2893.
- Ghoulam C, Fares K (2001) Effect of Salinity on Seed Germination and Early Seedling Growth of Sugar Beet (Beta Vulgaris L.). Seed Science and Technology 29: 357-364.
- Garg G (2010) Response in germination and seedling growth in Phaseolus mungo under salt and drought stress. Journal of Environmental Biology 31(3): 261-264.
- He XQ, Du C, Shao Z, Li Q (2009) Effect of Salt and Water Stress on Seed Germination of Dianthus Chinensis L. Academic Conference of Horticultural Science and Technology Proceedings 60-62.
- Oliveira N, BL de Steiner F, Honda GB, Machado JS (2016) Seed germination and early growth of physic nut seedlings under salinity stress. Scientia Agraria Paranaensis 15(4): 416-420.
- Kaur G, Kumar A (2016) Influence of Salinity Stress on Germination and Early Seedling Growth of Coriander (Coriandrum Sativum L.) Cultivars. International Journal of Science and Research 5(10): 69-48.
- Chauhan A, Rajput N, Kumar D, Kumar A, Chaudhry AK (2016) Effect of different salt concentration on seed germination and seedling growth of different varieties of oat (Avena sativa L.). International journal of information research and review 3(7): 2627-2632.
- Daneshnia F, Amini A, Chaichi MR (2016) Berseem clover quality and basil essential oil yield in the intercropping system under limited irrigation treatments with a surfactant. Agricultural Water Management. 164: 331-339.
- Winter E, Lauchli A (1982) Salt Tolerance of Trifolium alexandrinum L. Comparison of the Salt Response of alexandrinum.T. and pretense T. Australian Journal of Plant Physiology 9(2): 221-226.
- Ashkan A, Jalal M (2013) Effects of salinity stress on seed germination and seedling vigor indices of two Halophytic plant species (Agropyron elongatumand A. pectiniforme). International Journal of Agriculture and Crop Sciences 5(22): 2669-2676.
- Castroluna A, Ruiz OM, Quiroga AM, Pedranzani HE (2014) Effects of salinity and drought stress on germination, biomass and growth in three varieties of Medicago sativa L. Advances in Agricultural Research 18(1): 39-50.
- Mandic V, Krnjaja V, Bijelic Z, Tomic Z, Simic A, et al. (2014) Genetic variability of red clover seedlings in relation to salt stress. Biotechnology in Animal Husbandry 30(3): 529-538.
- Almansouri M, Kinet JM, Lutts S (2001) Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf. L). Plant and Soil 231(2): 243-254.
- Daneshnia F, Amini A, Chaichi MR (2015) Surfactant effect on forage yield and water use efficiency for berseemclover and basil in intercropping and limited irrigation treatments. Agricultural Water Management 160: 57-63.
- Rehman S, Harris PJC, Bourne WF, Wilkin J (1996) The effect of sodium chloride on germination and the potassium and calcium content of Acacies seeds. Seed Science and Technology 25(1): 45-57.
- Bu HY, Du GZ, Chen XL, Wang YF, Xu XL, et al. (2009) The evolutionary significance of seed germinability in an alpine meadow on the eastern Qinghai-Tibet Plateau. Arctic, Antarctic and Alpine Research 41(1): 97102.
- Wu FB, Zhang GP, Dominy P (2003) Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environmental and Experimental Botany 50(1): 67-78.
- Mitra S, VIS E, Kumar R, Plumb R, Fam M (2006) Wetting agents and cultural practices increase infiltration and reduce runoff losses of irrigation water. Biologia Bratislava 19(61): 353-357.
- Chaichi MR., Nurre P, Slaven J, Rostamza M (2015) Surfactant application on yield and irrigation water use efficiency in corn under limited irrigation. Crop Science Journal 55(1): 386-393.
- SAS Institute (1996) SAS System, 8th ed. SAS Inst, USA.
- Ahmad S, Ahmad R., Ashraf MY, Ashraf M, Waraich EA (2009) Sunflower (Helianthus annuus L.) response to drought stress at germination and seedling growth stages. Pakistan Journal of Botany 41: 647-654.
- Albuquerque FMCD, Carvalho NMD (2003) Effect of type of environmental stress on the emergence of sunflower (Helianthus annuus L.), soybean (Glycine max (L.) Merril) and maize (Zea mays L.) seeds with different levels of vigor. Seed Science Technology 31(2): 65-467.
- Soleyman A, Hesam M, Shahrajabian H (2012) Study of cold stress on the germination and seedling stage and determination of recovery in rice varieties. International Journal of Biology 4(4): 23-30.
- Maleki Farahani S, Mazaheri D, Chaichi M, Tavakkol Afshari R, Savaghebi G (2010) Effect of seed vigour on stress tolerance of barley (Hordeum vulgare) seed at germination stage. Seed Science Technology 38: 494507.
- Patane C, Cavallaro V, Avola G, DAgosta G (2006) Seed respiration of sorghum [Sorghum bicolor (L.) Moench] during germination as affected by temperature and osmoconditioning. Seed Science Research 16(4): 251-260.
- Ramagopal S (1990) Inhibition of seed germination by salt and its subsequent effect on embryonic protein synthesis in barley. Journal of Plant Physiology 136: 621-625.
- Meyer RF, Boyer JS (1981) Osmoregulation, solute distribution, and growth in soybean seedlings having low water potentials. Planta 151(5): 482-489.
- Parida AK, Das AB (2005) Salt tolerance and salinity effect of plants: a review. Ecotoxicology and Environmental Safety 60(3): 324-349.
- Panda SK, Khan MH (2009) Growth, oxidative damage and antioxidant responses in greengram (Vigna radiata L.) under short-term salinity stress and its recovery. Journal of Agronomy and Crop Science 195(6): 442-54.
- Daszkowska Golec A (2011) Arabidopsis seed germination under biotic stress as a concert of action of phytohormones. OMICS: A Journal of Integrative Biology 15(11): 763-774.
- Cavallaro V, Antonio Barbera AC, Maucieri C, Gimmaa G, Scalis C, et al. (2016) Evaluation of variability to drought and saline stress through the germination of different ecotypes of carob (Ceratonia siliqua L.) using a hydrotime model. Ecological Engineering 95: 557-566.
- Kader MA, SC Jutzi (2002) Temperature, osmotic pressure and seed treatments influence imbibition rates in sorghum seeds. Journal of Agronomy and Crop Science 188(4): 286-290.
- Ashraf M, Wahid S (2000) Time-course changes in organic metabolites and mineral nutrients in germinating maize seeds under salt (NaCl) stress. Seed Science and Technology 28(3): 641-656.
- Khodadad M (2011) An evaluation of safflower genotypes (Carthamus tinctorius L.), seed germination and seedling characters in salt stress conditions. African Journal of agricultural research 6: 1667-1672.
- Demie M, Kostka S, Boerth T, Franklin M, Ritsema C, et al. (2007) The effect of soil surfactants on soil hydrological behavior the plant growth environment, irrigation efficiency and water conservation. Journal of Hydrology and Hydromechanics 58(3): 142-148.
- Soldat DJ, Birl L, Wayne R (2010) Surfactants increase the uniformity of soil water content and reduce water repellency on sand-based golf putting greens. Soil Science 175(3): 111-117.
- Ghollarata M, Raiesi F (2007) The adverse effects of soil salinization on the growth of Trifolium alexandrinum L. and associated microbial and biochemical properties in a soil from Iran. Soil Biology and Biochemistry 39(7): 1699-1702.
- Kostka SJ, Cisar JL, Mitra S, Park DM, Ritsema CJ, et al. (2007) Irrigation efficiency Surfactants can save water and help maintain turfgrass quality. Golf Course Industry 19: 91-95.
- Orak A, Ates E (2005) Resistance to salinity stress and available water levels at the seedling stage of the common vetch (V Sativa L.). Plant, Soil and Environment 51: 51-56.
- Poulter RE, Duff AA, Bauer B (2009) Quantifying surfactant interaction effects on soil moisture and turf quality. Horticulture Australia Limited.
- Aflaki F, Sedghi S, Pazuki A, Pessarakli M (2017) Investigation of seed germination indices for early selection of salinity tolerant genotypes: A case study in wheat. Emirates Journal of Food and Agriculture 29(3): 222-226.
- Al Taisan WA (2010) Comparative Effects of Drought and Salt Stress on Germination and Seedling Growth of Pennisetum divisum (Gmel.) Henr. American Journal of Applied Sciences 7(5): 640-646.
- Amukali O, Obadoni BO, Mensah JK (2015) Effect of different NaCl on germination and seedling growth of Amaranthus hybridus and Celosia argentea. African Journal of Environmental Science and Technology 9(4): 301-306.
- Bahrani A, Haghjoo M (2011) Response of some wheat (Triticum aestivum L.) genotypes to salinity at germination and early seedling growth stages. World Applied Sciences Journal 16(4): 599-609.
- Cokkizgin A (2012) Salinity Stress in Common Bean (Phaseolus vulgaris L.) Seed Germination. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 40(1): 177-182.
- Ebadi Almas D, Bagherikia S, Mahdavi Mashaki K (2013) Effects of Salt and Water Stresses on Germination and Seedling Growth of Artemisia vulgaris (L.). International journal of agriculture and crop sciences 6(11): 762-765.
- El Shaieny AAH, Haleem A (2015) Seed germination percentage and the early seedling establishment of five (Vigna unguiculata L.) (Walp) genotypes under salt stress. European Journal of Experimental Biology 5(2): 22-32.
- El Harfi M, Hanine H, Rizki H, Latrache H, Nabloussi A (2016) Effect of Drought and Salt Stresses on Germination and Early Seedling Growth of Different Color-seeds of Sesame (Sesamum indicum). International Journal of Agriculture and Biology 18(6).
- Fetri M, Dargahikhoo A, Rajabi M (2014) Effect of drought and salinity tensions on germination and seedling growth of Common Yarrow (Achillea millefolium L.) in laboratory conditions. International Journal of Advanced Biological and Biomedical Research 2(2): 383-391.
- Hakim MA, Juraimi AS, Begum M, Hanafi MM, Ismail Mohd R., et al (2010) Effect of salt stress on germination and early seedling growth of rice (Oryza sativa L.). African Journal of Biology 9(13): 1911-1918.
- Hassanpouraghdam MB, Emarat Pardaz J, Farsad Akhtar N (2009) The effect of osmo priming on germination and seedling growth of Brassica napus L. under salinity conditions. Journal of Food Agriculture and Environment 7(2): 620- 622.
- Jamil M, Lee CC, Rehman SU, Lee DB, Ashraf M, et al. (2005). Salinity (NaCl) tolerance of Brassica species at germination and early seedling growth. Electronic Journal of Environmental, Agricultural and Food Chemistry 4: 970-6.
- Jorenush MH, Rajabi M (2015) Effect of Drought and Salinity Tensions on Germination and Seedling Growth of Artichoke (Cynara Scolymus L.). International Journal of Advanced Biological and Biomedical Research 3(3): 297-302.
- Nasri N, Saïdi I, Kaddour R, Lachaâl M (2015) Effect of Salinity on Germination, Seedling Growth and Acid Phosphatase Activity in Lettuce. American Journal of Plant Sciences 6: 57-63.
- Nasri N, Kaddour R., Mahmoudi H, Baatour O, Bouraoui N, et al. (2011) The effect of osmo priming on germination, seedling growth and phosphatase activities of lettuce under saline condition. African Journal of Agricultural Research. 10(65): 14366-14372.
- Sarker A, Hossain MdI, Abul Kashem Md (2014) Salinity (NaCl) tolerance of four vegetable crops during germination and early seedling growth.international Journal of Latest Research in Science and Technology 3: 91-95.
- Shrestha A, Cox R, Wu Y, Robles O, L deSouza L, et al. (2016) Moisture and Salt Tolerance of a Forage and Grain Sorghum Hybrid during Germination and Establishment. Journal of Crop Improvement. 30(6): 668-683.
- Soliman WS, El Shaieny AAH (2014) Effect of saline water on germination and early growth stage of five Apiaceae species. African Journal of agricultural research 9(7): 713-719.
- Torabi B, Ghehsareh AF (2013) Effect of Salt and Drought Stresses on Germination Components in Canola (Brassica napus L.). International Journal of Agriculture and Crop Sciences 5(15): 1642-1647.
- Wang WB, Kim BYH, Lee BHS, Kim CKY, Deng XP, et al. (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiology and Biochemistry 47(7): 570-577.
- Wu C, Wang Q, Xie B, Wang Z, Cui J, et al. (2011) Effects of drought and salt stress on seed germination of three leguminous species. African Journal of Biotechnology 10(78): 17954-17961.
- Yildirim E, Karlidag H, Dursun A (2011) Salt tolerance of physalis during germination and seedling growth. Pakistan Journal of Botany 43(6): 2673-2676.
- Zhang JT, MU CS (2009) Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus quinquenervius. Soil Science and Plant Nutrition 55(5): 685-697.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...














