Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4676
Research Article(ISSN: 2637-4676) 
Evaluation of Stripe Rust (Puccinia Striformis f. sp. Tritici) Resistance in Bread Wheat (Triticum aestivum L.) Genotypes in Ethiopia Volume 9 - Issue 3
Alemu Ayele*, Getnet Muche, Tamirat Negash, Lidiya Tilahun, Hawila Tesfaye, Daniel Kassa, Fikrte Yirga and Shumi Regassa
- Ethiopian Institute of Agricultural Reseasrch (EIAR) Kulumsa Agricultural Research Center Assela Ethiopia
Received: April 21, 2021; Published: May 17, 2021
Corresponding author: Alemu Ayele, Ethiopian Institute of Agricultural Reseasrch (EIAR) Kulumsa Agricultural Research Center Assela Ethiopia
DOI: 10.32474/CIACR.2021.09.000316
Abstract
Adult Plant Resistance (APR) based on partial resistance is an important and effective way to combat yellow rust (Puccinia striiformis) in wheat production. The objective of current research was planned to evaluate the response of 436 wheat (Triticum aestivum) genotypes against yellow rust resistance under field conditions during 2020 main cropping season. Over locations, Partial resistance screening was evaluated through Final Rust Severity (FRS), Area under Disease Progress Curve (AUDPC), Infection Rate (r), Coefficient of Infection (CI), Relative Area under Disease Progress Curve (rAUDPC) and field reaction have used for differentiating Adult plant resistances. Responses of four hundred thirty six genotypes, one hundred fourteen wheat lines were high adult plant resistance, two hundred thirty three lines were found to be intermediate adult plant resistant and two hundred eighty one were low adult plant resistance. With rAUDPC values over location twenty seven were 1-10 shown resistant, eighty seven lines were 11-30 categorized as moderately susceptible and three hundred twenty two genotypes exhibited susceptible response against yellow rust with more than 31-100 rAUDPC value. High values above 30 of rAUDPC showed greater severity of yellow rust on wheat genotypes while lower rAUDPC values indicated resistance to yellow rust. Fifty bread wheat genotypes that were selected based on overall agronomic performance (biomass, spike length, number of spikes/m2, tillering capacity, stalk strength or lodging resistance, shattering resistance and diseases resistance especially Septoria blotch and yellow rust. Three genotypes were EBW192345, EBW192346 and EBW192347 extraordinarily out performed evaluated materials phenotypically in terms of agronomic performance and diseases resistance over locations. The present study revealed that the lines were having enough diversity regarding slow rusting behavior and yellow rust resistance, ranging from immunity to partial resistant lines. Present research provided the resistant wheat lines to the breeders to incorporate in their breeding program against yellow rust.
Keywords: Bread Wheat; Yellow rust; Partial resistance; Adult Plant Resistance
Introduction
Stripe rust (Puccinia striiformis f. sp. tritici, Pst) is the most devastating rust disease that attacks much of global wheat production. The rapid emergence of virulent Pst races has overcome most of the known stripe rust resistance genes in wheat. Stripe rust of wheat is serious problem for wheat production worldwide and has reportedly caused significant yield losses in more than 60 countries (Chen, 2005).Epidemics of the disease can rapidly destroy leaf tissue and significantly reduce grain yield and quality. In most wheat-producing areas, yield losses caused by stripe rust range from 2.7 to 96.7% depending on the degree of susceptibility of the cultivar, timing of the initial infection, rate of disease development, areas of hotspot and duration of disease(Alemu Ayele and Getnet Muche, [1]). Currently, 80 yellow rust resistance (Yr) genes have been permanently named in wheat, including the recently mapped Yr79 (Feng et al., [2]) and Yr80 (Nsabiyera et al., [3]). Development and use of resistance genes in wheat breeding is the most effective, economic and environmental friendly approach for controlling stripe rust of wheat (Chen, [4]; 2007). Resistance to stripe rust is broadly categorized as: all stage resistance (also called seedling resistance), which can be detected at the seedling stage, but is also expressed at all stages of plant growth; and adult plant resistance (APR), which is expressed at later stages of plant growth. The cultivation of resistant varieties remains the most economic and environmentally preferable method to manage this disease. Some of the resistance genes are effective at seedling stage and they are race specific. Several of these genes may become ineffective due to the emergence of new virulent races and also because of rapid evolution and adaptation of pathogen Kolmer et al., [5]. In contrast, others are effective through the adult plant stage and are referred to as slow rusting genes and they are race non-specific provide durable resistance or a broad spectrum of races. Therefore, a cultivar that only has slow rusting resistance to leaf rust will display susceptible infection type response throughout the entire lifecycle of the plant (Fahmi et al., [6]). Although several studies have been carried out to assess stripe rust resistance in different wheat genotypes in Ethiopia, many of them were based on race specific resistance. Adult plant resistance can be measured in the field by recording disease severity at weekly intervals and then calculating the area under disease progress curve (AUDPC) Wilcoxson, (1981). The present study was thus designed to assess the levels of slow rusting resistance in national bread wheat genotypes to yellow rust under field conditions.
Materials And Methods
Plant materials
Four hundred thirty six bread wheat genotypes that were obtained from Ethiopian Institute of Agricultural Research (EIAR) Kulumsa Agricultural research Center which is national wheat research program coordinating center in Ethiopia, evaluated under field conditions at Kulumsa main station, Bekoji and Meraro experimental sites for 2020 main cropping season. The lines were sown small adjacent plots of two rows per plot, with each row of 1m length separated by 0.2 m with a distance of 0.4 m between entries which were selected due to severely affected hotspot areas for yellow rust in Ethiopia. A mixture of Morocco, PBW343, Kubsa and Digalu which are a super susceptible wheat cultivars, were sown around entries as spreader rows and also to serve as an adult plant susceptible check.
Disease scoring
Disease Scoring was made three times at Kulumsa and Bekoji, and four times at Meraro experimental stations at fourteen days interval, starting when susceptible spreader rows reached 20% severity according to the Modified Cobb Scale (Paterson et al., [7]).
Final rust severity (FRS)
Final rust severity (FRS) was used to classify wheat genotypes into different group such as 1-30 percent as moderately resistant, 31-50percent as moderately susceptible and 51-90 percent as susceptible.
Coefficient of infection (CI)
Coefficient of infection was calculated by using data on disease severity and host reaction by multiplying the severity value by a value of 0.10, 0.4, 0.8 or 1.00 for host response rating of R, MR, MS or S, respectively and was used to classify genotypes in to three groups such as 1-20 High adult plat resistance, 21-40 intermediate and 41-100 low adult plant resistance (Pathan and Park, [8]).
AUDPC and rAUDPC value; - were calculated as Van der Plank, 1963 and, Milus and Line, 1986
AUDPC=(N1(X1+X2)/2+(N2(X2+X3))/2+(N3(X3+X4))/2 Where, X1, X2, X3 and X4 are rust intensities recorded on first, second, third and fourth recording date and N1 is interval day between X1 and X2 N2 is interval day between X2 and X3, N3 is interval day between X3 and X4
rAUDPC=((line AUDPC)/(Susceptible AUDPC))100
Results And Discussion
Final rust Severity
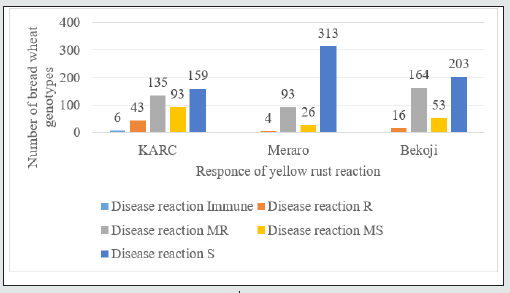
Diverse field reactions ranging from resistance (R) to susceptible (S) responses were observed at the Kulumsa, Bekoji and Meraro experimental sites. The final rust severities of the genotypes and their infection types are presented in Figures 1and 2. Final rust severity represents the cumulative result of all resistance factors during the progress of epidemics (Parlevliet and van Omeren, 1975). Based on final rust severity, the tested wheat genotypes were grouped into three groups of slow rusting resistance, that is, high, intermediate and low levels of adult plant resistance(APR) having 1-30 and 31- 50 and 51-100% FRS, respectively. At Bekoji experimental station two hundred thirty three wheat genotypes displayed disease severities of up to 30%. Of these sixteen genotypes had resistant(R), one hundred sixty four had moderately resistant (MR), fifty three moderately susceptible (MS) responses while two hundred three showed susceptible(S) field reactions. On the other hand, at Kulumsa six, forty three, one hundred thirty five, ninety three and one hundred fifty nine genotypes were showed immune, R, MR, MS and S field reaction to the yellow rust. Despite the heavy yellow rust disease pressure at Meraro, four and ninety three genotypes remained in the first group, exhibiting final rust severities ranging from 1 to 30%, with compatible R and MR responses and are of great importance to achieve effective breeding for durable resistance to yellow rust (Parlevliet, [9]; Nzuve et al., [10]). According to Nzuve et al. [10]), the available resistance genes in these materials overcame the yellow rust virulence in the field and led to statistically low disease severities despite the compatible host-pathogen reactions. Previously, Ali et al. [11], Li et al. [12], Tabassum [13], Abebele GM, et al. [14], Safavi [15] and Heena Attri and Tuhina Dey [16] also used final rust severity to assess slow rusting behavior of wheat lines. On the other hand fifty eight genotypes showed final rust severities between 31 and 50% and two hundred sixty four genotypes showed at all experimental stations and were regarded as possessing high levels of slow rusting resistance. The immune response on these tested genotypes could be as a result of hypersensitive responses; resistance often breaks down due to the development of new races of the pathogen. A suitable breeding strategy like the use of inter-specific and remote crosses or even the direct transfer of these resistances through backcrosses could be used to improve the adopted but highly susceptible wheat varieties being grown in Ethiopia (Bartos et al., [17]). On the other hand, the susceptible check, PBW343, Digalu, Kubsa and Morocco displayed the highest disease severities of 90% with completely susceptible (S) responses at Meraro, Bekoji and Kulumsa experimental stations, indicating that an acceptable epidemic pressure was established over the seasons for field experiments.
Average coefficient of infection
Table 1: Final rust severity, Response reaction and coefficient of infection of the selected bread wheat genotypes at three locations.
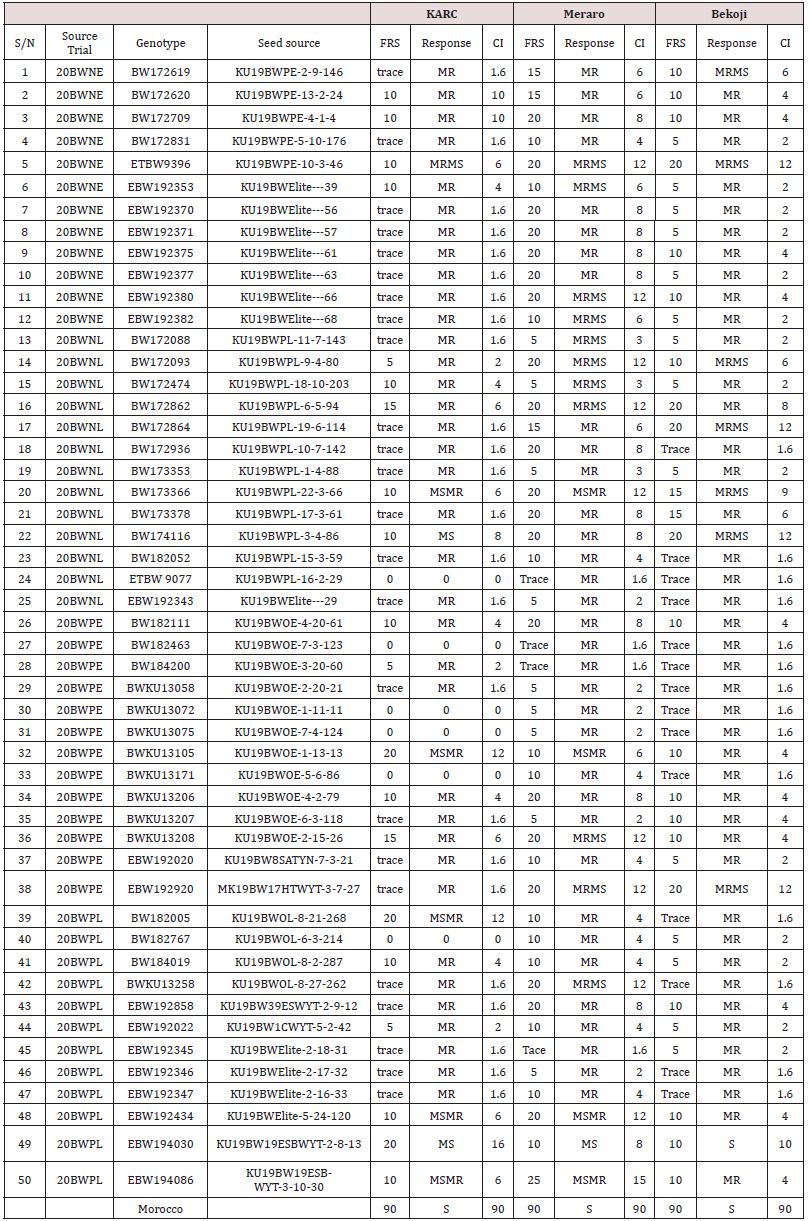
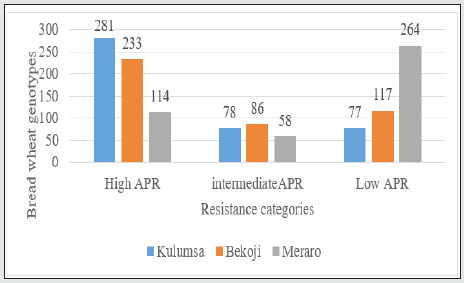
The data on disease severity and host reaction were combined to calculate CI (Figure 3). According to Ali et al. [18], lines with CI values of 0-20, 21-40, 41-60 were regarded as possessing high, moderate and low levels of slow rusting resistance, respectively. In the present study, all the test genotypes over locations ninety nine genotypes showed CI values between 0 and 20 were designated as having a high level of slow rusting. It was, therefore, concluded that these genotypes had a great potential to be used as a resistance sources against yellow rust. Only forty eight genotypes, had CI values of 21 to 40, designated as having moderate levels of slow rusting resistance and two hundred eighty eight genotypes had a CI value of more than 40, designated as having low levels of slow rusting and grouped as susceptible genotypes. Many earlier researchers such as Patil et al. [19]; Pathan and Park [28] and Draz et al. [20] also appraised slow rusting resistance to wheat leaf rust using coefficient of infection and reported the presence of different partial resistance conferring genes in wheat lines(Table 1).
Figure 3: Average coefficient of infection of wheat genotypes under resistance category to yellow rust.
Area under disease progress curve (AUDPC) and relative area under disease progress curve (RAUDPC)
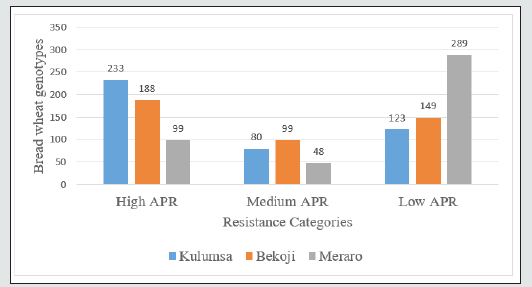
Disease progress curve is a better indicator of disease expression over time (Van der Plank, [21]). Therefore, selection of genotypes having lower RAUDPC values is acceptable for practical purposes. The tested wheat genotypes were categorized into three distinct groups for slow rusting resistance, based on the rAUDPC values. Wheat genotypes exhibiting rAUDPC values up to 30% of the check were grouped as having high level of partial resistance, consisted of 114, 291, 195 wheat genotypes, while those having rAUDPC values to 70% of the check were grouped as moderately resistant genotypes included 195, 130 and 201 genotypes at Meraro, Kulumsa and Bekoji experimental stations respectively (Figure 4). Of the wheat genotypes under group resistant over location were 114 genotypes exhibiting low disease pressure and high level of partial resistance to yellow rust showed R to MR and 130 showed MRMS types of infection in the field. According to Parlevliet [9], Brown et al. [22], Singh et al. [5], and Kaur and Bariana [23] the genotypes which had MS infection type may be carrying durable resistance genes, such as slow rusting resistance. These wheat genotypes first shown rust infection and sporulation but the final host reaction was characterized as chlorotic and necrotic lesions. Subsequently, the disease progression remained slower and highly retarded among these genotypes. Such partially resistant lines could highly delay evolution of new virulent races of the pathogen because multiple point mutations are extremely rare in normal circumstances (Schafer and Roelfs, [24]; Ali et al., [25]; Tsilo et al.,[26]. Likewise, despite the MS infection type exhibited on moderately slow rusting genotypes, rust developed slowly as indicated by their AUDPC values.. Other researchers have also reported variation among different wheat lines for slow rusting resistance using AUDPC (Patil et al., [19]; Draz et al., [20]) (Table 2).
Table 2: AUDPC and rAUDPC values of the selected bread wheat germ-plasms at three Experimental stations
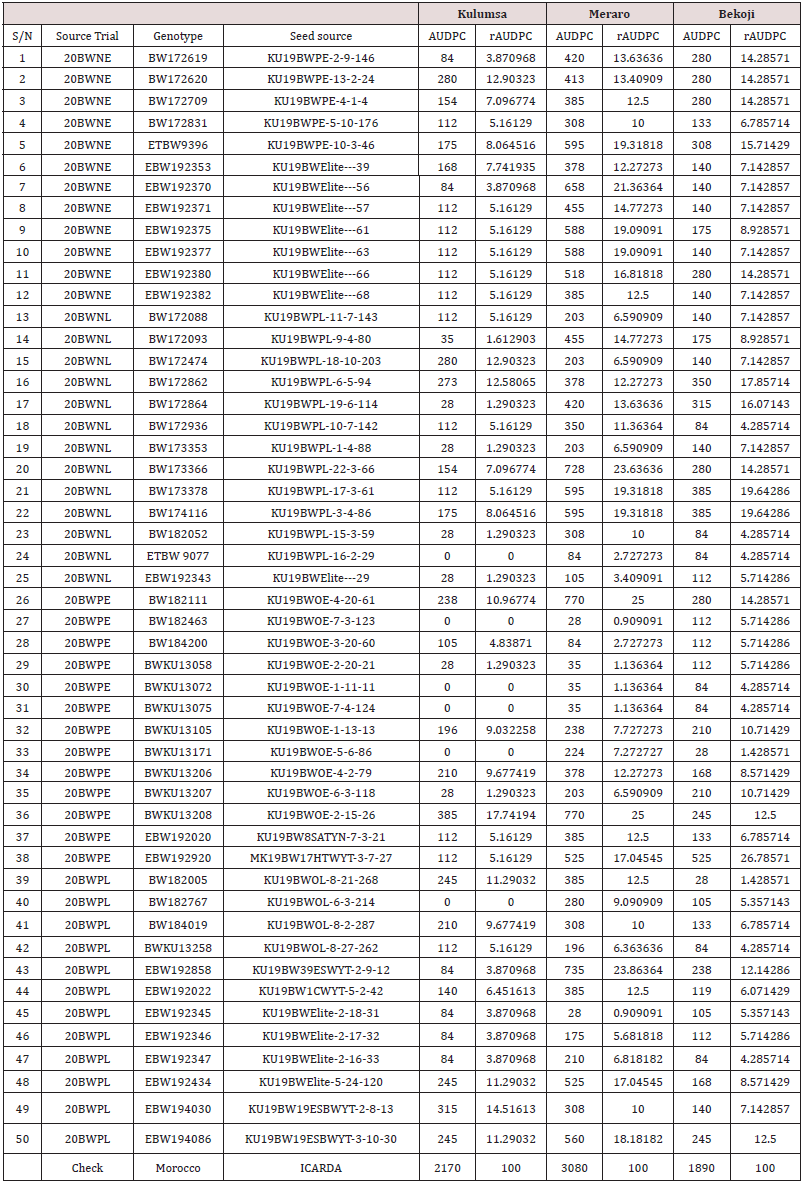
Figure 4: Level of Partial Resistance of wheat genotypes in relative area under disease progress curve to yellow rust.

Conclusion
The wheat genotypes showed variation in resistance reaction, ranging from immunity to slow rusting resistance. Most of the evaluated genotypes over locations exhibited better performance under high disease pressure shown by susceptible check. The four hundred forty six tested genotypes 114 exhibited lower levels of FRS (< 30% with R-MR responses), coefficient of infection (< 20) and rAUDPC less than 30% indicating a high level of slow rusting resistance [27-31].
Forty-five at Meraro and Fifty at Bekoji Bread wheat genotypes that were selected based on overall agronomic performance (Biomass, spike length, number of spikes/m2, tillering capacity, stalk strength or lodging resistance, shattering resistance and Disease’s resistance especially Septoria blotch and yellow rust. Three genotypes were EBW192345, EBW192346 and EBW192347 extraordinarily out performed evaluated materials phenotypically in terms of agronomic performance and diseases resistance at all locations. Hence, these genotypes can be verified across locations and registered as a variety in the coming year if they are already released somewhere in the world. Likewise, all selected materials shall be included under bread wheat national variety Trial (BWNVT) in the coming season. Moreover, these genotypes can also be included in multipurpose crossing blocks in order to improve weakness of commercial varieties.
It is concluded that from the current research that, the slow rusting genotypes identfied from this study with better levels of adult plant resistance to be exploited for durable resistance in Ethiopian wheat breeding program. However, further testing for stability over years and locations for yellow rust along with other desirable characters must be made to abate yield losses and to ensure food security before approval.
Acknowledgements
Accelerating Genetic Gain in Wheat (AGGW) is sincerely thanked for financial support of the study. The Ethiopian Institute of Agricultural Research (EIAR) is acknowledged for hosting the field research. The all-round support provided by the wheat rust research team of Kulumsa Agricultural Research Center of Ethiopia is highly appreciated.
References
- Alemu Ayele, Getnet Muche (2019) Yield Loss Assessment in Bread Wheat Varieties Caused by Yellow Rust (Puccinia striiformis f. sp. tritici) in Arsi Highlands of South Eastern Ethiopia. American Journal of BioScience 7(6): 104-112.
- Feng JY, Wang MN, See DR, Chao SM, Zheng YL, et al. (2018) Characterization of novel gene Yr79 and four additional QTL for all stage and high-temperature adult-plant resistance to stripe rust in spring wheat PI 182103. Phytopathology 108(6): 737-747.
- Vallence Nsabiyera , Harbans S Bariana, Naeela Qureshi, Debbie Wong, Matthew J Hayden, et al. (2018) Characterization and mapping of adult plant stripe rust resistance in wheat accession Aus27284. Theoretical and Applied Genetics: 131(7): 1459-1467.
- Chen XM (2005) Epidemiology and control of stripe rust [Puccinia striiformis f. sp.tritici] on wheat.Canadian Journal of Plant Pathology, 27: 314-337.
- Kolmer JA, Singh RP, Garvin DF, Viccars L, William HM (2008) Analysis of the Lr 34/Yr18 rust resistance region in wheat germplasm. Crop Science 48(5): 1841-1852.
- Fahmi AI, Nazim M, Khalifa SZ, ElOrabey WM (2005) Genetics of adult plant resistance to leaf rust in Egyptian wheat. Egyptian Journal of Phytopathology 33: 1-10.
- Paterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Canadian Journal of Research 26(5): 496-500.
- Pathan AK, Park RF (2006) Evaluation of seedling and adult plant resistance to leaf rust in European wheat cultivars. Euphytica 149: 327-342.
- Parlevliet JE (1988) Resistance of the Non-Race-Specific Type. In “The Cereal Rusts”, Vol. II. Diseases, Distribution, Epidemiology and Control, Academic Press, Orelando.
- Nzuve FM, Bhavani S, Tusiime G, Njau P, Wanyera R (2012) Evaluation of bread wheat for both seedling and adult plant resistance to stem rust. Afr J Plant Sci 6(15): 426-432.
- Ali S, Jawad S, Shah A, Ibrahim M (2007) Assessment of wheat breeding lines for slow yellow rusting (Puccinia striformis west. tritici). Pak J Biol Sci 10(19): 3440-3444.
- Li ZF, Xia XC, He ZH, Li X, Zhang LJ, et al. (2010) Seedling and slow rusting resistance to leaf rust in Chinese wheat cultivars. Plant Dis 94: 45-53.
- Tabassum S (2011) Evaluation of advance wheat lines for slow yellow rusting (Puccinia striiformis f. sp. tritici). J Agric Sci 3: 239-249.
- Getnet Muche Abebele, Merkuz Abera Admasu, Bekele Hundie Agdu, 2020. Field Evaluation of Bread Wheat (Triticum aestivum L) Genotypes for Stripe Rust (Puccinia striiformis W.) Resistance in Arsi Highlands of Oromia Region, South -Eastern-Ethiopia. J Plant Pathol Microbiol 11(10): 520.
- Safavi SA, Ahari AB, Afshari F, Arzanlou M (2010) Slow rusting resistance in 19 promising wheat lines to yellow rust in Ardabil, Iran. Pakistan Journal of Biological Science13(5): 240- 244.
- Heena Attri, Tuhina Dey (2021) Screening of Stripe Rust Resistance in Bread Wheat (Triticum aestivum L.) Genotypes. Int J Curr Microbiol App Sci 10(01): 1236-1244.
- Bartos P, Sip V, Chrpova J, Vacke J, Stuchlikova E, Blazkova V, Sarova J, Hanzalova A (2002) Achievements and prospects of wheat breeding for disease resistance. Czech J. Genet. Plant Breed 38(1): 16-28.
- Ali S, Shah SJA, Khalil IH, Raman H, Maqbool K, et al. (2009) Partial resistance to yellow rust in introduced winter wheat germplasm at the north of Pakistan. Aust J Crop Sci 3(1): 37-43.
- Patil VS, Hasabnis SN, Narute TK, Khot GG, Kumbhar CT (2005) Rusting behaviour of some wheat cultivars against leaf rust under artificial epiphytotic conditions. Indian Phytopathol 58(2): 221-223.
- Draz IS, Abou-Elseoud MS, Kamara AM, Alaa-Eldein OA, El-Bebany AF, et al. (2015) Screening of wheat genotypes for leaf rust resistance along with grain yield. Ann Agric Sci 60(1): 29-39.
- Van der Plank JE (1963) Plant diseases. Epidemic and Control. Academic Press, New York. pp. 17-27.
- Brown WMJ, Hill JP, Velasco VR (2001) Barley yellow rust in North America. Annu. Rev. Phytopathol. 39: 367-384.
- Kaur J, Bariana HS (2010) Inheritance of adult plant stripe rust resistance in wheat cultivars Kukri and Sunco J Plant Pathol 92(2): 391-394.
- Kaur J, Bariana HS (2010) Inheritance of adult plant stripe rust resistance in wheat cultivars Kukri and Sunco J Plant Pathol 92(2): 391-394.
- Ali S, Shah SJA, Maqbool K (2008) Field-based assessment of partial resistance to yellow rust in wheat germplasm. J. Agric. Rural Dev 6(1): 99-106.
- Tsilo TJ, Jin Y, Anderson JA (2010) Identification of Flanking Markers for the Stem Rust Resistance Gene Sr6 in Wheat. Crop Sci 50(5): 1967-1970.
- Milus EA, Line, RF (1986) Gene action for inheritance of durable, high temperature, adult plant resistance to stripe rust in wheat. Phytopathology 76: 435-441.
- Pandey HN, Menon TCM, Rao MV (1989) A single formula for calculating area under disease progress curve. Rachis 8: 38-39.
- Parlevlie JE, Van-Ommeren A (1975) Partial resistance of barley to leaf rust, Puccinia hordei. II. Relationship between field trials, micro plot tests and latent period. Euphytica 24: 293-303.
- Singh RP, Huerta-Espino J, William HM (2005) Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. Turk J Agric For 29: 121-127.
- Wilcoxson RD, Skovmand B, Atif AH (1975) Evaluation of wheat cultivars ability to retard development of stem rust. Annu Appl Biol 80(3): 275-281.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...