
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4676
Research Article(ISSN: 2637-4676) 
Efficacy of Selected Attractants for Monitoring the Populations of the Redbay Ambrosia Beetle Xyleborus Glabratus Eichhoff (Coleoptera: Scolytidae) and Other Bark Beetles in the Florida Panhandle Volume 1 - Issue 3
Latasha D Tanner1, Lambert HB Kanga1*, Muhammad Haseeb1, Leroy Whilby2 and Oghenekome U Onokpise1
- 1Center for Biological Control, Florida A&M University, USA
- 2Florida Department of Agriculture and Consumer Services, Florida A&M University, USA
Received: February 15,2018; Published: February 22,2018
Corresponding author: Lambert H B Kanga, Center for Biological Control, Florida A&M University, USA
DOI: 10.32474/CIACR.2018.01.000112
Abstract
The redbay ambrosia beetle, Xyleborus glabratus Eichhoff (Coleoptera: Scolytidae) is a non-native insect first discovered in the United States in 2002 in Port Wentworth, Georgia. This beetle has a direct impact on the natural forest ecosystem because it vectors the fungus Raffaelea lauricola, which causes laurel wilt disease, a vascular disease of trees in the family Lauraceae, such as redbay, sassafras, camphor, silkbay, pondspice, bay laurel, the endangered pondberry and the economically important avocado. Originally from Southeast Asia the beetle has spread to coastal forests of Alabama, Florida, Georgia, Mississippi, North Carolina and South Carolina. This study surveyed previously un-surveyed areas of the Florida Panhandle, the Apalachicola National Forest usinga) A mixture of manuka and phoebe oil, b) Ethanol gel attractants and c) Hand sanitizer.
The redbay ambrosia beetle was not detected in the Apalachicola National Forest; however, another 2,394 specimens of beetles belonging to the tribe Xyleborini and related tribes of the Scolytinae were found. Of these, more than 90% belonged to introduced (invasive) species. Gel ethanol was significantly more attractive to ambrosia beetles than the manuka and phoebe oil mixture. When hand sanitizer was substituted as a source of ethanol, no significant differences were found between the numbers of beetles captured by the manuka and phoebe oil mixture and by hand sanitizer. Thus, hand sanitizer was as attractive as the commercial product. The presence of high numbers of invasive beetles suggests that an even larger number of fungi are being introduced and they are potential threats to the trees in the Apalachicola National Forest's ecosystems. Hand sanitizer attractant could be used as an alternative to gel ethanol as it is cost-effective, affordable, and sustainable
Keywords: Redbay ambrosia beetle; Monitoring; Gel ethanol; Manuka and phoebe oil; Hand sanitizer; Apalachicola national forest; Bark beetle
Introduction
The redbay ambrosia beetle, Xyleborus glabratus Eichhoff, (Coleoptera: Scolytidae) is a wood-boring insect that vectors the fungus responsible for a vascular laurel wilt causing high mortality of trees within the Lauraceae family in the southeastern United States [1]. The beetle carries the symbiotic fungal pathogen in its mandibular mycangium [2]. Once introduced into the living tree,the fungus blocks the xylem, thus producing a disease syndrome termed "laurel wilt" (LW) which eventually leads to tree death [3]. Ambrosia beetles are members of the insect tribe Xyleborini, have a symbiotic relationship with a fungus and are known for attacking various woody plants, causing some limb and stem dieback and sometimes plant death [4-6]. Redbay ambrosia beetle adults bore through the bark and into the xylem where they lay eggs and inoculate the wood with the symbiotic fungus; then, adults and larvae cultivate and feed on ambrosia fungi that grow in the galleries [7]. Invasive fungus-forming ambrosia beetles (Xyleborus, Xylosandrus) pose a growing threat to forest ecosystems and fruit industries [8].
Native species of Persea (Lauraceae) species appear to be referred hosts, and laurel wilt disease is responsible for high mortality of redbay [P. borbonia (L.) Spreng.], swampbay [P palustris (Raf.) Sarg.], and sassafras [Sassafras albidum (Nuttall) Nees] in Alabama, Florida, Georgia, Mississippi, North Carolina and South Carolina [9,10]. In addition, the avocado (Persea americana Mill) crop in Florida, the United States and Mexico is threatened by this serious disease [11,12]. Thus, there is an urgent need for assessing the density of the populations and the distribution of these beetles. Development of monitoring/management tools for the redbay ambrosia beetle is contingent upon identification of effective attractants. The standard method of monitoring ambrosia beetles include use of Lindgren funnel traps baited with Manuka and Phoebe oil [13,14]. However, recent field tests conducted in Florida indicated that Manuka attractants were not specific in attraction of X. gIabratus and had limited longevity. Phoebe oil attractants were found to be highly effective for capture of X. glabratus [11], but unfortunately, they are no longer available due to depletion of source trees in the Brazilian rain forest. The beetle is attracted to the odor of Raffaelea [15], but there is no evidence of an aggregation pheromone or a response to ethanol (the standard lure for other ambrosia beetles). Bolts of redbay are proven attractants suggesting host tree volatiles are the primary attractants [8]. Because the Apalachicola National Forest (the largest of Florida forests) has never been surveyed for the presence of the redbay ambrosia beetle, this study aims to provide useful insights in the composition of ambrosia beetles and potential threats to the trees in the Apalachicola National Forest's ecosystems.
Materials and Methods
Site selection
The sites were chosen in consultation with the personnel of Cooperative Agricultural Pest Survey (CAPS). These sites were easily accessible to campers who might import contaminated firewood from other regions of Florida, believed to be the major route of dispersal of beetle [16]. The three campsites selected were: (a) Camel Lake near Bristol (GPS coordinates 30.256102, -84.994955) located off of NF-105; (b) Cotton Landing (coordinates 30.05394, -85.064049) located off of NF-102A, and (c) Wright Lake/Hickory Landing (coordinates 29.991739, -84.978304) located off of NF- 101 near Sumatra.
Survey procedure and field trials
The surveys covered two experimental periods; the first survey began on October 2011 and ended on February 2012 and the second one was conducted from March 2012 to August 2012. Two commercial attractants were tested:
a) Manuka and phoebe oil mixture (Synergy Semiochemicals, Burnaby, BC) which is a recognized attractant for XyIeborus gIabratus, and
b) Gel ethanol lures (Contech Inc. Delta, BC, Canada) recognized as general bark beetle attractants. Results indicated that gel ethanol was an effective attractant, but difficult to obtain commercially.
Therefore, hand sanitizer (Purell® Hand Sanitizer, Purell, Akron, OH, USA) was also tested to see if it was effective in attracting beetles. The attractants were evaluated in three campsites (Camel Lake near Bristol, Cotton Landing and Wright Lake/Hickory Landing). Different attractant treatment regimens were tested in three experimental units or blocks. The treatment applied to each experimental unit was unchanged throughout the 10 months of the study. In the experimental units 1, 2 and 3, manuka and phoebe oil mixture was used for treatment 1, gel ethanol for treatment 2 and hand sanitizer for treatment 3.
These treatments (attractants) were applied as per the manufacturer's instructions. For treatment 3, a latex laboratory glove was filled with commercially available hand sanitizer from a retail distributor, sealed with twine, and three pinholes were placed near the top to allow the scent to disperse. Two Lindgren funnel traps (10 funnels/trap) were set at each site and randomly assigned a treatment. The attractant was changed monthly and all ambrosia beetles were collected at a scheduled time interval. Samples were preserved in cups containing propylene glycol and later placed in vials of ethanol with dates and location. Specimens collected were taken to our laboratory in Tallahassee, Florida, and sorted, then separated and identified, and later sent to CAPS laboratory in Gainesville, Florida, for a second verification of the identification of the specimens. The percentage of ambrosia beetle collected in each trap was used to assess the effectiveness of each treatment in the experimental unit.
Data Analysis
Data on the numbers of ambrosia beetles collected were analyzed by treatment, location, and date using a repeated measure analysis [17]. Treatments (attractants) were modeled as fixed effects; date, location and date-by-treatment interactions were modeled as random effects. Locations and the dates were used to provide the replications for the treatment effects, and treatments and date provided the replications to test the fixed effects of the location. Similarly, the treatments and location provided replications to the fixed effects of the date. A Tukey's studentized range test was used to compare treatment means. Data were log transformed [log (x+1)] to satisfy the assumptions of normality before analysis. For all statistical tests α=0.05.
Results and Discussion
Diversity of species collected
Table 1: List of species collected and identified during the experimental periods from October 2011 to February 2012.
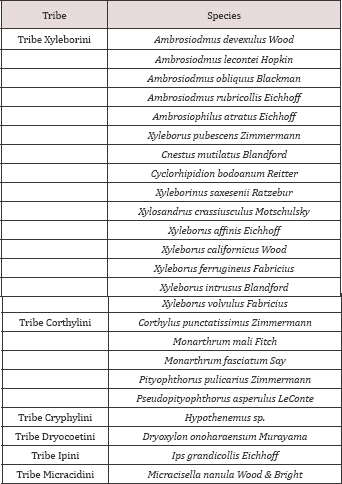
Overall, a total of 2,394 specimens of ambrosia beetles representing 25 species and 15 genera were collected from the Apalachicola National Forest, but Xyleborus glabratus were not found. Ofthose collected, 1,865 belonged to Xyleborini, the ambrosia beetle tribe containing the redbay ambrosia beetle and many other forest species (Table 1). During the first survey period, 217 specimens were collected, the relative abundance of these species ranged from 0.46% to 51.61% (Table 2). The dominant species was Xyleborinus saxesenii (51.61%). There were also four subdominant species which included Xyleborus affinis (9.20%), X. ferrugineus (8.76%), and X. volvulus (7.83%), all belonging to the tribe Xyleborini (Figure 1). However, the specimens of Hypothenemus sp. (11.01%) belong to the related tribe Cryphalini (Table 2). There were relatively low total numbers of beetles collected in winter; results for the spring and summer sampling produced a total of2.177 specimens ( Table 3).
Table 2: Species of scolytine ambrosia beetles collected in the Apalachicola National Forest, October 2011 to February 2012, arranged alphabetically. (N=217).
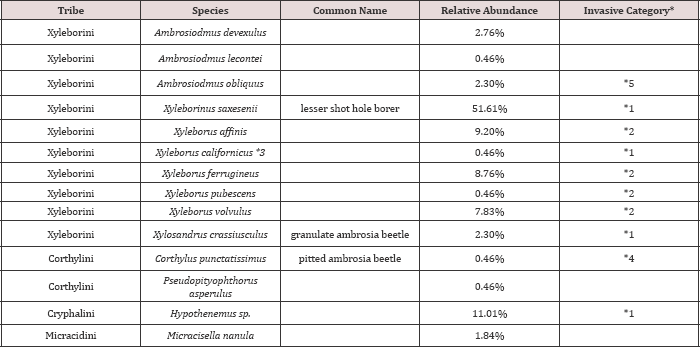
*1-exotic beetles established in USA, or *2-intercepted at ports in USA, 1984 to 2008; *3-Xyleborus californicus was recently synonymized with Cyclorhipidion bodoanum; *4-record from CISEH (2010); *5-possibly introduced
Figure 1: Number of specimens of dominant species of scolytine ambrosia beetles collected in Apalachicola National Forest from October 2011 to August 2012. N&3x003D;2,394.
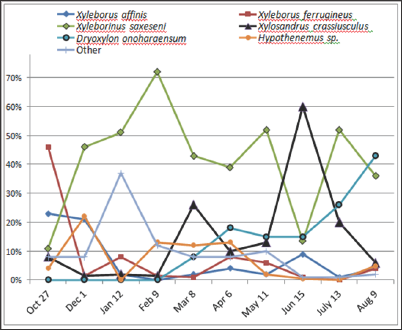
The seasonal patterns of occurrence of these species varied over time and between species (Figure 2). The relative density of populations of Xyleborinus saxesenii increased from 11% in October (fall) to 72% in February (late winter); in contrast, beetle populations of Xyleborus ferrugineus were highest at the beginning of the survey period, then decreased from 48% in October to 10% in January, and later showed a small increase in April (7.6%) and declined from May through August. Xyleborinus saxesenii was the most abundant representative of Xyleborini in every sample except in October 2011 and June 2012. In June, the abundance of X. saxesenii was less than that of the winter species XyIosandrus crassiuscuIus which increased from 5% of total beetles in February (spring) to 60% in June (summer), or in terms of numbers from one specimen in February to 16 specimens in May to 517 specimens in June. In contrast, the number of XyIeborinus saxesenii decreased from 42% to 12% over the same period (Figure 2).
Table 3: Species of scolytine ambrosia beetles collected in the Apalachicola National Forest, October 2011 to August 2012 arranged by order of relative abundance. (N=2,394).
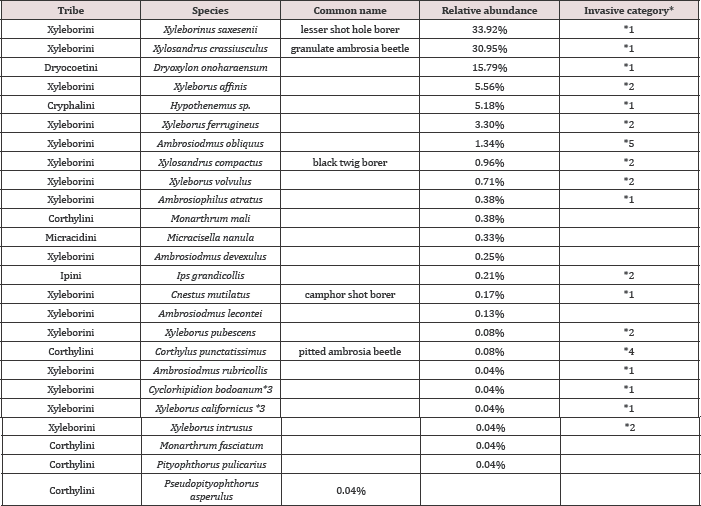
*1-exotic beetles established in USA, or *2-intercepted at ports in USA, 1984 to 2008; *3-Xyleborus californicus was recently synonymized with Cyclorhipidion bodoanum; *4-record from CISEH (2010); *5-possibly introduced.
Figure 2: Seasonal distribution and relative abundance per date for dominant species of scolytine +ambrosia beetles, Apalachicola National Forest, October 2011 to August 2012. N=2,394.
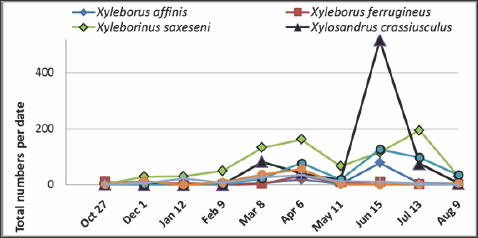
Based on the tabular summaries of species intercepted at American ports the highest percent of species (97.4%) of ambrosia beetles collected were invasive species [18]. Our data suggested that forests were changing as invasive insects import diseases in the absence of natural enemies [8,19]. Our data also indicated four newly collected species or new county records from the Apalachicola National Forest (Table 3). These species include Corthylus punctatissimus which was collected at Camel Lake in February using ethanol gel and in March using Manuka/ Phoebe oil. Cnestus mutilatus was originally collected using ethanol gel in April and again using hand sanitizer in May and July, all at Camel Lake, and in June at the same site using Manuka Phoebe oil. Cnestus mutilatus is a pest of grapes and many trees and is also a known vector of the recently described fungus Ambrosiella beaver [20]. Xyleborus intrusus was collected at Cotton Landing using hand sanitizer attractant in May, and Monarthrum fasciatum was collected using hand sanitizer at Cotton Landing in June. The relative abundance of the species collected during October 2011 to August 2012 (Table 3) indicated that the most dominant species were Xyleborinus saxeseni(33.92%), Xylosandrus crassiusculus (30.95%) and Droxylon onoharaensum (15.79).
Effectiveness of attractants for the ambrosia beetle
Table 4: Comparative efficacy of gel ethanol and Manuka /Phoebe Oil attractants to species of Xyleborini during the survey period: October 2011 to April 2012.
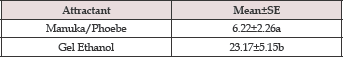
Means in column are not significantly different if followed by the same letter (Tukey's test, SAS Institute, 2006).
Data on the total number of ambrosia beetles belonging to Xyleborini collected in the first six months indicated that gel ethanol was significantly more attractive to the ambrosia beetles than was a mixture of Manuka and Phoebe oil (F=5.57; df=1,10; P=0.0401) (Table 4). In the last four months (May to August 2012), commercial hand sanitizer was tested as attractant instead of gel ethanol. The numbers of beetle collected using hand sanitizer were not significantly different from those using Manuka and Phoebe oil mixture (F= 0.05; df=1,4; P=2070.8275) ( (Table 5). The time effect (a measure of within-treatment variability) was significant (F=29.57; df=3,12; P <0.0001).
Table 5: Comparative efficacy of hand sanitizer and Manuka/ Phoebe Oil attractants to total specimens of Xyleborini collected during the survey period: May to August 2012.
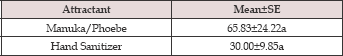
Means in column are not significantly different if followed by the same letter (Tukey's test, SAS Institute, 2006).
Among the three major species of ambrosia collected during the experimental periods in the Apalachicola National Forest 386 specimens of X. saxesenii were attracted to the Manuka/Phoebe oil while 426 to ethanol; 562 specimens of X. crassiusculus were collected in Manuka/Phoebe oil but 179 specimens in ethanol, and for Xyleborus ferrugineus 29 in Manuka/Phoebe and 50 in ethanol. These results are more in agreement with researchers who showed Xyleborinus saxeseni attracted to ethanol [21] and Xylosandrus crassiusculus responded to odors from wounded redbay trees in flight intercept traps [9]. Our data clearly indicated the Xylosandrus crassiusculus has a peak flight activity in June which is similar to the findings of Werle [22] from Mississippi but not those from South Carolina where the beetles were more active in August and September [9]. Perhaps the differences in seasonal abundance of these species may be found in the growth and availabilities of different host trees.
Using Lindgren funnels with different attractants, 25 species of ambrosia beetles were identified in the Apalachicola National Forest over the period from October 2011 to August 2012. The majority of beetles collected were invasive species. Two invasive species, Xyleborinus saxesenii and Xylosandrus crassiusculus, were the most abundant species in the samples. The redbay ambrosia beetle (Xyleborus glabratus) was not found, but considering the recent reports of laurel wilt disease in counties west and north of the Apalachicola National Forest, it may be present or the fungus may be carried by another of these species. Carillo [23] showed that five of the species present in the forest (Xyleborinus saxeseni, Xyleborus affinis, X. ferrugineus, X. volvulus, Xylosandrus crassiusculus) were capable of vectoring the laurel wilt fungus. In 1983, Xylosandrus crassiusculus was first recorded from Florida in a locality adjacent to the study sites in the Apalachicola National Forest [24] and was common in the area by 1994 [25]. It vectors symbiotic fungi that cause heavy damage to many species of trees; it is also capable of vectoring the same fungus that is carried by the redbay ambrosia beetle [26]. Xyleborinus saxesenii is a known pest in ornamental nurseries [27], and may also carry the laurel wilt fungus [28].
The distribution of four other species was extended to the Apalachicola National Forest in Liberty County, Florida. The dominant species, Xylosandrus crassiusculus showed a strong seasonal peak in summer in adult populations and Xyleborinus saxesenii was present on every sampling date. The attractants were a mixture of Manuka and Phoebe oil used over the entire period of study and two formulations of ethanol: a standard commercial gel ethanol for six months and hand sanitizer for four months. The survey results indicated that gel ethanol was significantly more attractive to beetles than the Manuka/Phoebe oil mixture. When hand sanitizer was substituted as a practical source of ethanol, there was no significant difference between hand sanitizer and the Manuka/Phoebe oil mixture. This provides a practical and economical method for routine monitoring of ambrosia beetles. This is the first report on the use of hand sanitizer as an alternative to Manuka/Phoebe oil mixture.
Acknowledgement
We are grateful to Ms. Janice Peters and Dr. Manuel Pescador (Florida A&M University) for providing useful discussions and reviews of the manuscript. We also thank Ms. Sabrina Hayes (Florida A&M University) for her technical assistance with this study. This study was supported in part by a Grant # 2013-32100-08905 from the USDA-NIFA McIntire-Stennis Cooperative Forestry Program.
References
- Crane JH, Smith JA (2010) Homeowner detection ofand recommendations for mitigating redbay ambrosia beetle-laurel wilt disease on redbay and avocado trees in the home landscape. Univeristy of Florida, USA.
- Harrington TC, Fraedrich SW, Aghayeva DN (2008) Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 104: 399-404.
- Fraedrich SW, Harrington TC, Rabaglia RJ, Ulyshen MD, Mayfield AE III, et al. (2008) A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis 92(2): 215-224.
- Rabaglia RJ, Dole SA, Cognato AI (2006) Review of the American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occurring north of Mexico, with an illustrated key. Ann Entomol Soc Am 99(6): 1034-1056.
- Atkinson TH, Peck SB (1988) Annotated checklist of the bark and ambrosia beetles (Coleoptera:Scolytidae) of tropical southern Florida. Florida Entomol 7: 313-329.
- Atkinson TH, JL Foltz, MD Connor (1994) Flight patterns of phloem and wood boring Coleoptera (Scolytidae, Platypodidae, Curculionidae, Buprestidae, Cerambycidae) in a north Florida slash pine forest. Environ Entomol 17: 259-265.
- Thomas MC (2007) Two Asian ambrosia beetles recently established in Florida (Curculionidae: Scolytinae). Dept Plant Industry, Florida Department of Agriculture and Consumer Services.
- Hulcr J, Dunn RR (2011) The sudden emergence of pathogenicity in insect-fungus symbiosis threatens native forest ecosystems. Philos Trans R Soc Lond Ser 278(1720): 2866-2873.
- Hanula JL, Sullivan B (2008) Manuka oil and phoebe oil are attractive baits for Xyleborus glabratus (Coleoptera: Scolytinae), the vector of laurel wilt. Environ. Entomol 37(6): 1403- 1409.
- Gramling JM (2010) Potential effects of laurel wilt on the flora of North America. Southeastern Naturalist 9(4): 827-836.
- Kendra PE, Sanchez JS, Montgomery WS, Okins KE, Niogret J, et al. (2011) Diversity of Scolytinae (Coleoptera: Curculionidae) attracted to avocado, lychee, and essential oil lures. Florida Entomol 94(2): 123-130.
- FDACS (2012) Disease Threatens Health of Avocado Trees, a $13 Million Industry in South Florida. Florida Department of Agriculture and Consumer Services.
- Rabaglia R, Duerr D, Acciavatti R, Ragenovich I (2008) Early detection and rapid response for non-native bark and ambrosia beetles. US Dep Agric Forest Service, Washington, USA.
- Kendra PE, Montgomery WS, Niogret J, Pena J, Epsky ND (2010) Attraction of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) to avocado, lychee, and essential oil lures. Florida Entomol Soc Ann.
- Hulcr J, Mann RR, Stelinski LL (2011) The scent of a partner: ambrosia beetles are attracted to volatiles from their fungal symbionts. J Chem Ecol 37(12): 1374-1377.
- Mayfield A, Eickwort JM (2010) Risk assessment of the movement of firewood within United States. USA.
- (2006) The SAS system for Windows. North Carolina, USA.
- Haack RA, Rabaglia RJ (2013) Exotic bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) in the United States: potential and current invaders. pp. 48-74. In JE Pena (edn.) (2013) Potential Invasive Pests of Agricultural Crop Species. CABI International, Wallingford, UK.
- Ploetz RC, Hulcr J, Wingfield MJ, de Beer ZW (2013) Destructive tree diseases associated with ambrosia and bark beetles: black swan events in tree pathology? Plant Disease 97(7): 856-872.
- Six DL, Stone WD, de Beer ZW, Woolfolk SW (2009) Ambrosiella beaveri sp nov associated with an exotic ambrosia beetles, Xylosandrus mutiliatus (Coleoptera: Curculionidae, Scolytinae), in Mississippi, USA. Antonie van Leeuwenhoek 96(1): 17-29.
- Atkinson TH, Foltz JL, Connor MD (1988) Flight patterns of phloem and wood-boring Coleoptera (Scolytidae, Platypodidae, Curculionidae, Buprestidae, Cerambycidae) in a north Florida slash pine forest. Environ Entomol 17(2): 259-265.
- Werle RT, Sampson BJ, Oliver JB (2012) Diversity abundance of seasonality of ambrosia beetles (Coleoptera: Curculionidae) in southern Mississippi. Midsouth Entomol 5: 1-5.
- Carillo D, Duncan RE, Ploetz JN, Campbell AF, Ploetz RC et al. (2014) Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Path 63(1): 54-62.
- Chapin JB, Oliver AD (1986) New records for Xylosandrus and Xyleborus species (Coleoptera: Scolytidae). Proc Entomol Soc Wash 88: 680-683.
- Mizell RF, Braman S, Sparks B, Hudson W (1994) Outbreak of the Asian ambrosia beetle, Xylosandrus crassiusculus (Motschulsky), is cause for concern. Proc South Nurs Assoc Res Conf 39: 191-193.
- Harrington TC, Aghayeva DN, Fraedrich SW (2010) New combinations of Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 111: 337-361.
- Ranger CM, Reding ME, Gandhi KJK, Oliver JB, Schultz PB et al. (2011) Species dependent influence of (-) a-pinene on attraction of ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) to ethanol-baited traps in nursery agro-ecosystems. J Econ Entomol 104(2): 574-579.
- Harrington TC, Fraedrich SW (2010) Quantification of propagules of the laurel wilt fungus and other mycangial fungi from the redbay ambrosia beetle, Xyleborus glabratus. Phytopathology 100(10): 1118-1123.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...














