
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4676
Research Article(ISSN: 2637-4676) 
Effect of invigoration treatments on artificially aged Brassica napus L seeds Volume 9 - Issue 5
Ehsan Khalid1*, Faryal Fatima1, Sadar Uddin Siddqui1, Muhammad Musharaf Latif2, Ravi Prakash Jha3, Waseem Khalid4 and Muhammad Zubair Khalid4**
- 1Seed Preservation Lab, PGRI, NARC, Islamabad-45500, Pakistan
- 2Seed Physiology Lab, Department of Agronomy, University of Agriculture, Faisalabad 38040, Pakistan
- 3Department of Community Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- 4Department of Food Science, Government College University Faisalabad
Received: January 17, 2022; Published: January 27, 2022
Corresponding author: Ehsan Khalid, Seed Preservation Lab, PGRI, NARC, Islamabad-45500, Pakistan
DOI: 10.32474/CIACR.2022.09.000325
Abstract
Brassica napus L. (canola seeds) is one of the major edible oil sources in all over the world and is cultivated mainly for its oil rich seed. This study was assessed the utility to invigorate artificially deteriorated canola seeds (expose to 40°C and 100% Relative humidity for 24 hours) by hydro-priming (distilled water 100ml), halo-priming (NaCl), osmo-priming (KNO3), nutrient-priming (KH2PO4) solutions of 1, 2, 3 and 4% respectively for 8 hours and compared with control. Priming treatments cause significant germination percentage increase in hydro-priming (70%) with maximum seedling vigor index I and II (594.42 and 2.80). In NaCl 1% it was 66.67% as compare to control (48.33%). In KNO3 priming it was maximum at 4% (58.33%) and in KH2PO4 at 1% (60%). Results of experiment conclude that hydro-priming induced rapid and uniform germination with increase in seedling vigor, seedling length, fresh and dry weight.
Keywords: Canola; Deteriorated seeds; Hydro-priming; Vigor
Introduction
Brassica napus L. commonly known as Rapa, oil seed Rapa, Rappi, Rapa seed. Bright yellow flowering member of family Brassicaceae (mustard or cabbage family). This family contains 375 genera and 3200 species includes crops, ornamental plants, and many weeds. The members of the genus are collectively known as cruciferous vegetables, cabbages, or mustards etc. Common types of Brassica used for food include cabbage, cauliflower, broccoli, Brussels sprouts, and some types of seeds. Oil seed rapa was formed 7500 years ago by hybridization between B.rapa and B.oleraceae, followed by chromosome doubling, a process known as allopolyploid. The name derives from the Latin for turnip, rapa or rapum, and is first recorded in English at end of 14th century. Older writers usually distinguished the turnip and Rapa by the objectives round and long rooted respectively. Brassica napus L. is one of the major sources of edible oil all over the world and is cultivated mainly for its oil rich seed. The 3rd largest source of vegetable oil in world. Brassica napus L. is sparingly grown for young leaves used as forage for livestock feed, and as source of rapeseed oil. Rape oil used in food industry, as an illuminant and lubricant, and for soap manufacture. Residual rapeseed cake, though low in food value, used as livestock feed. Rapeseed oil has potential market in detergent lubrication oils, emulsifying agents, polyamide fibers, and resins, and as a vegetable wax substitute. Rapeseed and mustard are rich source of oil and contains 44% to 46% good quality oil. In addition, its meal has 38- 40% protein.
In Pakistan the rape and mustard crop was grown on an area of about 242.6 thousand hectares with a seed production of 230.7 thousand tons with the average yield of 951 kg per ha. In Punjab, Sindh, KPK and Balochistan it is grown on area of 159.4, 51.3, 14.0 and 17.9 thousand hectares respectively. The average yield in Punjab is 986 kegs per hectare. In Sindh its yield is 1072 kegs per hectare, KPK and Balochistan with yield of 493 and 654 kegs per hectare respectively (Agricultural Statistics of Pakistan, [1]). Seed aging is process which results in delaying germination, reduction in germination rate and sometime total loss in viability (Priestly, [2]). Stress that high temperature and relative humidity is responsible for seed aging process and found that seed aging also leads to various cellular and metabolic alterations including loss of membrane integrity degradation of DNA, reduction in primary metabolism (El-Maarouf-Bouteau et al., [3]). Seed aging is highly associated with storage conditions and seeds of different plant species show different rate of seed aging under same storage conditions (Walters et al., 2005). Artificially aged seeds can be invigorated by providing some invigoration techniques such as priming (presowing hydration treatment), seed coating technologies and seed conditioning (Taylor et al., [4]). Seed priming is widely adopted as a tool to improve seed germination under optimal and non-optimal conditions (Caliskan et al., 2011). In Brassica napus L., hydropriming, hormonal priming and osmo-priming has been found to improve growth parameters in normal and adverse conditions (Basra et al., [5]).
Seed germination and seedling growth are considered the most important phase for seedling establishment and determining successful crop production (Uniyal et al., 1998). Hydration dehydration (Priming) is an efficient pre-sowing treatment (Nath et al., 1991). Production potential of Canola crop is high as compare to its average yield. One prime important constrain regarding low production of oil seed is seed quality. By invigoration techniques germination can be increased (Taylor et al., [4]). Priming is valuable process for improving germination and uniformity of heterogeneously matured seed lot (Olouch et al., 1996). Seed germination delays under stress and priming increase germination and seedling growth (Kaya., 2006). Seed priming has been successfully demonstrated to improve germination and emergence, particularly in seeds of vegetables and small seeded grasses (Angadi., 2002). Brassica napus L. seeds storage go through summer season. This is a reason of loss in seeds vigour and viability. In this study Brassica napus L. seeds was artificially aged at high temperature and relative humidity conditions and conduct invigoration treatments to determine the effect of invigoration techniques on germination percentage, seedling root length, shoot length, seedling fresh and dry weight.
Materials and methods
To investigate the effect of accelerated aging and invigoration by priming on the seed germination of rape seed (Brassica napus L.) cultivar was studied in Plant Seed Preservation Lab, PGRI, NARC, Islamabad, Pakistan in 2017. Seeds were collected from National Gene Bank, PGRI, NARC, Islamabad.
Accelerated aging
Accelerated aging test was conducted by exposing the seeds to 100% relative humidity at 40°C and 50°C for three different time durations (24, 48 and 72 hours) to estimate reduction in germination up to 50%. For further study seeds were exposed to 100% relative humidity at 40°C for 24 hours.
Seed priming
Various priming treatments viz., hydro-priming (Distilled water), halo-priming (NaCl), osmo-priming(KNO3), nutrient-priming (KH2PO4) was studied on seed invigoration in comparison with control as following, T0: control, T1: hydro-priming, T2: NaCl (01%), T3: NaCl (02%), T4: NaCl (03%), T5: NaCl (04%), T6: KNO3 (01%), T7: KNO3 (02%), T8: KNO3 (03%), T9: KNO3 (04%), T10: KH2PO4(01%), T11: KH2PO4(02%), T12: KH2PO4(03%), T13: KH2PO4(04%). All these priming treatments were imposed by directly soaking seed material (24 hours aged at 40°C) for 08 hours at room temperature (20-22°C). After priming treatment, seeds were given three surface washings with distilled water and surface dried at room temperature of 20-22°C. Following observations were recorded.
Seed Germination
In germination test between papers method was used. Seeds were placed on surface of double sheet of paper towel, which were moistened with distilled water. The seed were covered with another sheet of paper towel (VICTORY paper towel, 22*23cm). The rolled sandwich were placed in plastic beaker and covered with plastic bag. Placed at incubator (25±02°C) for 08 days. The seed whose radical is equal to the size of seed was considered as germinated. Final count was made on 08th day. Germination % was calculated on base of normal seedlings (Anonymous, 1989).
Final Germination (%)= (Germinated seeds)/(Total seed)×100
Seedling length
Seedling length was estimated in three replications following the standard method (Gupta, 1993). Normal seedlings were picked randomly from the germinated seeds from each replication. The shoot and root length were measured from the collar region, where shoot joins with root. The seedling length is calculated as the sum of shoot and root length. Subsequently the mean was used for further analysis.
Seedling fresh weight
Seedling dry weight was estimated in three replications following the standard method (Gupta, 1993). Normal seedlings were picked randomly from the germinated seeds from each replication. Fresh weight was recorded.
Seedling dry weight
Seedling dry weight was estimated in three replications following the standard method (Gupta, 1993). Normal seedlings were picked randomly from the germinated seeds from each replication. Seedlings were dried at room temperature for 24 hours. Then place in oven at 121 ± 02°C for 02 hours. The dry weight was recorded after cooling in desiccator containing silica gel. Subsequently the mean was used for further analysis.
Seed vigor index
Quantitative measurement of seed vigor index 1 and 2 were calculated based on germination (%), seedling length (cm) and dry weight (g) using following formula,
Seed vigor index 1 = Germination % * Seedling length (cm) (Abdul-Baki and Anderson, [6]).
Seedling vigor index 2 = Germination% * Seedling dry weight (g) (Perry, [7])
Statistical analysis Experiment was laid out in Completely Randomized Design (CRD. Collected data was analyzed by using Statistix 8.1 (Steel et al., [8]).
Results
Brassica napus seeds were artificially aged at different temperature (40°C and 50°C) and high relative humidity (100%) and different durations (24, 48 and 72 hours) to reduce actual germination (71.67%) up to 50%. At 40°C temperature, 100% RH for 24 hours, seeds germination reduces to 49.01%. According to the results, all studied parameters were affected by invigoration techniques (Priming treatments). Germination percentage of hydropriming was maximum (70%) as compare to the control (48.33%) and it was near to the germination of seeds before aging (71.67%). In halo-priming increase in germination was 18.34% at NaCl 01% with the germination of 66.67%. In osmo- priming maximum germination percentage was 58.33% at KNO3 04% and in nutrient-priming it was 60% at KH2PO4 01% (Figure 1).
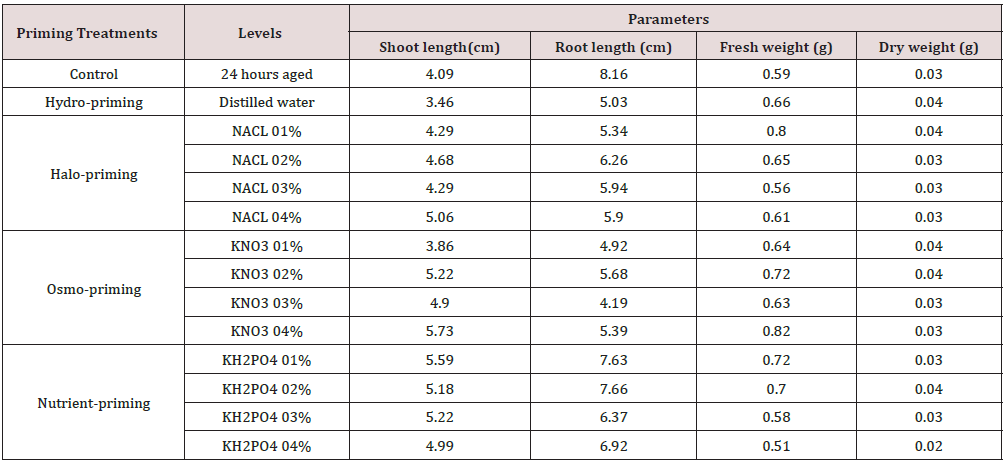
High seedling vigour I was recorded in KH2PO4 at 01 and 02% (793.26 and 748.85 respectively) followed by followed by KNO3 04% (648.58), NaCl 01% (641.84) (Figure 2). Seedling vigour index II was maximum at NaCl 01% (2.9) followed by hydro-priming (2.8), KH2PO4 at 02% (2.1) and at KNO3 04% it was 1.8 (Figure 3). Maximum shoot length was recorded in halo-priming KNO3 04% (5.73 cm) followed by nutrient-priming KH2PO4 01% (5.59 cm) as compare to control (4.09 cm). Minimum shoot length was in hydropriming treatment and it was 3.46cm. Root length is negatively influenced among different priming treatments. Maximum root length was recorded in Control (8.16 cm) and minimum was in KNO3 03% (4.19 cm). Maximum fresh weight was in halo-priming KNO3 04% (0.82 g) as compare to control (0.59 g). Minimum was in KH2PO4 04% (0.51 g). Seedlings dry weight was in range from 0.02-0.04 g in all invigoration treatments (Table 1).
Figure 2: Seedling vigour index I of control and invigorated artificially aged Brassica napus L. seeds.

Figure 3: Seedling vigour index II of control and invigorated artificially aged Brassica napus L. seeds.

Table 1: Shoot length, Root length, seedling fresh and dry weight of control and invigorated artificially aged Brassica napus L. seeds.
Discussion
In this study, Brassica napus seeds were artificially aged at 40°C temperature, 100% relative humidity for 24 hours and germination was reduced from 71.67% to 49.01%. High relative humidity and temperature deteriorate seeds quality during storage (Afzal et al., 2017). Seed quality is deteriorated in adverse conditions and the rate of deterioration accelerated with increase in temperature and relative humidity (Bradford et al., 1993). According to the results of priming, all studied parameters were affected significantly by priming treatments. Germination percentage of hydropriming was 70% as compare to the control (48.33%). In halo-priming, 18.34% germination percentage increase was observed at NaCl 01%. In osmo- priming maximum germination percentage was at KNO3 04% (58.33%) and it was in nutrient-priming at KH2PO4 01% (60%) (Fig. a). It is also reported in literature that priming treatments accelerate germination percentage of canola seeds. Priming induced rapid and uniform germination of canola seeds, which resulted in rapid emergence of seedlings. These results are supported by the previous study on canola (Zheng et al., [9]). Canola seeds responded to different priming treatments and seed yield was increased due to priming. Hydro priming performed better than other treatments. Primed seeds maintained their increased vigor by six months of low temperature storage (Shahzad et al., [5]). Hydropriming is a simple and useful technique for enhancing seedling emergence rate and percentage.
These effects can improve seedling establishment and field performance (Ghassemi-Golezani et al., [10]). High seedling vigour I was recorded in KH2PO4 at 01 and 02% (793.26 and 748.85 respectively) followed by followed by KNO3 04% (648.58), NaCl 01% (641.84) (Figure 2). Seedling vigour index II was maximum at NaCl 01% (2.9) followed by hydro-priming (2.8), KH2PO4 at 02% (2.1) and at KNO3 04% it was 1.8 (Figure 3). Increase in moisture effect quality that ultimately reduced seed vigour (Murthy et al., [11]; Shelar et al., [12]). Previous study shows that high rate of seed deterioration is because of high moisture conditions that resulted in seed vigour loss (Sharma and Meshram, [13-20]). Maximum shoot length was recorded in halo-priming KNO3 04% (5.73 cm) followed by nutrient-priming KH2PO4 01% (5.59 cm) as compare to control (4.09 cm). Minimum shoot length was in hydro-priming treatment and it was 3.46cm. Root length is negatively influenced among different priming treatments. Maximum root length was recorded in Control (8.16 cm) and minimum was in KNO3 03% (4.19 cm) [21-27]. Maximum fresh weight was in halo-priming KNO3 04% (0.82 g) as compare to control (0.59 g). Minimum was in KH2PO4 04% (0.51 g). Seedlings dry weight was in range from 0.02-0.04 g in all invigoration treatments (Table 1).
Conclusion
Seed quality (viability and vigour) which is deteriorated in high humidity and temperature conditions can be invigorated with help priming treatment. This study conclude that hydro-priming induced rapid and uniform germination with increase in viability as well as vigour of artificially deteriorated Brassica napus seeds.
Acknowledgement
The authors are highly acknowledged to Plant Seed Preservation Lab, PGRI, NARC and National Gene Bank, PGRI, NARC, Islamabad for providing valuable inputs to accomplish this research.
References
- (2013-2014) Agricultural Statistics of Pakistan p: 59-60.
- Priestley DA (1986) Seed Ageing: Implications for seed storage and persistence in the soil. New York, NY: Cornell University Press.
- El-Maarouf Bouteau H, Mazuy C, Corbineau F, Bailly C (2011) DNA alteration and programmed cell death during ageing of sunflower seed. Journal of Experimental Botany 62(14): 5003-5011.
- Taylor AG, Klein DE, Whitlow TH (1988) SMP: solid matrix priming of seeds. Scientia Horticulturae 37(1-2): 1-11.
- Basra SM, Ullah EH S A N, Warriach EA, Cheema MA, Afzal I (2003) Effect of storage on growth and yield of primed canola (Brassica napus) seeds. International journal of agriculture and biology 5(2): 117-120.
- Abdul-Baki A A, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria 1. Crop science 13(6): 630-633.
- Perry DA (1978) Report of the vigor test committee 1974-1977. Seed Sci. Technol 6: 159-181.
- Steel RGD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics: A biometrical approach. WCB/McGraw Hill Book Co., New York, USA.
- Zheng GH, Wilen RW, Slinkard AE, Gusta LV (1994) Enhancement of canola seed germination and seedling emergence at low temperature by priming. Crop Science 34(6): 1589-1593.
- Ghassemi-Golezani K, Aliloo AA, Valizadeh M, Moghaddam M (2008) Effects of different priming techniques on seed invigoration and seedling establishment of lentil (Lens culinaris Medik). Journal of Food Agriculture and Environment 6(2): 222.
- Murthy UN, Kumar PP, Sun WQ (2003) Mechanisms of seed ageing under different storage conditions for Vigna radiata (L.) Wilczek: lipid peroxidation, sugar hydrolysis, Maillard reactions and their relationship to glass state transition. Journal of Experimental botany 54(384): 1057-1067.
- Shelar VR, RS Shaikh, AS Nikam (2008) Soybean seed quality during storage: A review. Agric. Rev, 29(2): 125-131.
- Sharma K, Meshram NM (2006) Bioactivity of essential oils from Acorus calamus Linn. and Syzygium aromaticum Linn. against Sitophilus oryzae Linn. in stored wheat. Biopesticide International 2: 144-152.
- Abdolahi M, Andelibi B, Zangani E, Shekari F, Jamaati-e-Somarin S (2012) Effect of accelerated aging and priming on seed germination of rapeseed (Brassica napus L.) cultivars. International Research Journal of Applied and Basic Sciences 3(3): 499-508.
- Afzal I, Bakhtavar MA, Ishfaq M, Sagheer M, Baributsa D (2017) Maintaining dryness during storage contributes to higher maize seed quality. Journal of Stored Products Research 72: 49-53.
- Bradbeer JW (2013) Seed dormancy and germination. Springer Science & Business Media.
- Bradford KJ, Tarquis AM, & Durán JM (1993) A population-based threshold model describing the relationship between germination rates and seed deterioration. Journal of Experimental Botany 44(7): 1225-1234.
- Çaliskan O, Mavi, K, Polat A (2012) Influences of presowing treatments on the germination and emergence of fig seeds (Ficus carica L.). Acta Scientiarum. Agronomy 34: 293-297.
- Farhoudi R, Sharifzadeh F (2006) The effects of NaCl priming on salt tolerance in canola (Brassica napus L.) seedlings grown under saline conditions. Indian Journal of crop science 1(1and2): 74-78.
- Gupta PC (1993) Seed vigour testing. Handbook of seed testing. New Delhi 242, 249.
- Heshmat O, H A Saeed, K Fardin (2011) The improvement of seed germination traits in canola (Brassica napus L.) as affected by saline and drought stress. Journal of Agricultural Technology 7(3): 611-622.
- Jamil M, Rha ES (2007) Gibberellic acid (GA3) enhance seed water uptake, germination and early seedling growth in sugar beet under salt stress. Pakistan Journal of Biological Sciences 10(4): 654-658.
- Maroufi K, Farahani HA, Moaveni P (2011) Effects of hydropriming on germination in rapeseed (Brassica napus L.). Advances in Environmental Biology, Pakistan 5(8): 2208-2211.
- Mir-Mahmoodi T, Golizadeh SK, Khaliliqhdam N, Yazdanseta S (2014) The effect of salicylic acid on rate germination and seedling establishment on rapeseed (Brassica napus L.). International Journal of Agriculture Innovations and Research 2(6): 2319-1473.
- Mohammadi GR, Amiri F (2010) The effect of priming on seed performance of canola (Brassica napus L.) under drought stress. American-Eurasian Journal of Agricultural and Environmental Science 9(2): 202-207.
- Takayanagi K, Harrington JF (1971) Enhancement of germination rate of aged seeds by ethylene. Plant Physiology 47(4): 521-524.
- Verma R, Vijay D, Gupta CK, Malaviya DR (2014) Seed quality enhancement of oat (Avena sativa L.) varieties through priming. Range Management and Agroforestry 35(1): 144-150.
















.png)
.jpg)

