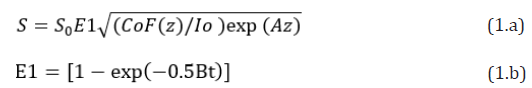Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4579
Mini Review(ISSN: 2637-4579) 
Kinetics and efficacy of photo-activated crosslink for tissue-engineering Volume 2 - Issue 4
Jui-Teng Lin*
- New Vision Inc. Taipei, Taiwan
Received: June 11, 2018; Published: July 19, 2018
Corresponding author: Jui-Teng Lin, New Vision Inc. Taipei, Taiwan, Email: jtlin55@gmail.com
DOI: 10.32474/OAJBEB.2018.02.000145
Abstract
We will review the kinetics, applications and efficacy of light-activated crosslink. The crosslink efficacy is influenced by multiple factors including: the applied light intensity, exposure period and dose, the initial concentration profiles of photosensitizers (PS) and oxygen, the quantum yield of the PS triplet state, the kinetic rate constants of PS (in type-I) and oxygen concentration (in type- II). Lower light intensity (I0) and higher PS concentration (C0) provides higher efficacy, but also suffers more toxicity to the cells. Higher light intensity (I0) may accelerate the crosslink but has lower efficacy (for the same dose). Therefore, optimal ratio of C0/ I0 is required for accelerated and safe crosslink. Safety issue also limits the maximum dose can be applied to the collagen, besides the limitation of PS concentration. Three important issues are presented: improvement of the crosslink speed (via higher light intensity) and efficacy of crosslink, and the role of photosensitizer (photo initiator) concentration.
Keywords: Lasers, Crosslink, Photodynamic Therapy, Tissue Engineering, Cancer Therapy, Ophthalmology
Introduction
Lasers have been used for various medical procedures such as dermatology and cosmetic surgery, wound healings, nerve stimulation, dentistry and ophthalmology applications for vision corrections and corneal deceases and many other therapeutic procedures [1-3]. Combining the nanoparticles, diode lasers have been also used for cancer therapy, bio-sensing, bio-imaging, drug delivery and diagnostics of cancer cells [4-8]. Lights (lasers or LED) are also used to activate cross-linking to stabilize collagen in human corneas [9-12], biomaterials for tissue engineering [13-18] and hydrogels for nanomedicine and drug delivery [19]. Fundamentals and applications of medical lasers for various clinical procedures were reviewed recently by Lin [3]. Laser spectra of UV (190-400) um, visible (400-700) nm, near-IR (700- 2900) nm, and mid-IR (3-5) um having various penetration depths (from determine the procedures are thermal or non-thermal or combined effect. invasive or noninvasive. Diode lasers have been widely used in many surgical procedures including soft tissue cutting, coagulation and cancer thermal therapy. Combining the nanoparticles and photosensitizers, diode lasers have been also used for cancer diagnosis and therapy [4,5]. Tissue-engineering using scaffold-based procedures have been attracting increasing attention [13-19], where chemical modification of gelatin has been reported to improve its mechanical properties by crosslinking or polymerization with UV [14-16] or visible light [17] to produce gels or high-molecular-weight polymers. A scaffold provides an environment for cell attachment, proliferation, differentiation, and drug delivery with high-loading efficiency at specific sites [13,14]. The goal of a photo-click hydrogel system is to identify key reaction parameters to enable fast gelation (<2 minutes), minimal photoinitiator- induced toxic response, and tunable hydrogel elasticity. In this mini-review article, we will focus on the kinetics of cross-linking to stabilize collagen in hydrogel biomaterials for tissue engineering [13-19]. we will present 3 important issues: improvement of the crosslink speed (via higher light intensity) and efficacy of crosslink, and the role of photosensitizer (photo initiator) concentration.
Materials and Methods
The selection of photosensitizers
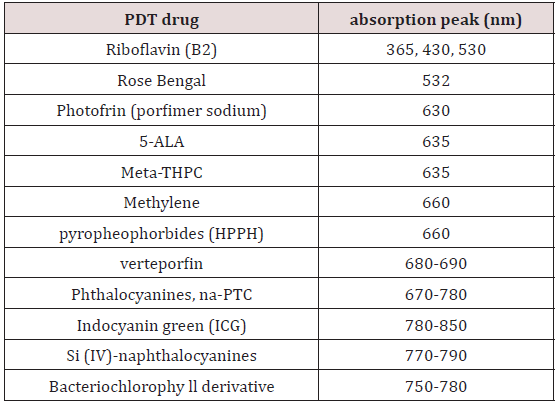
Photodynamic therapy (PDT) uses photosensitizers activated by lights (lasers or LEDs) for various clinical procedures. As shown in Table 1, various photosensitizers are available for the absorption of light (LED or lasers) in UV (365-400) nm, visible (430-700) nm, and near-IR (700-1000) nm. High-brightness LED in UV (365 nm) and visible (500-680) nm are also available for PDT using 5-ALA as the photosensitizer. In addition, riboflavin and Rose Bengal are used for the treatment of corneal diseases.
Photochemical Kinetics
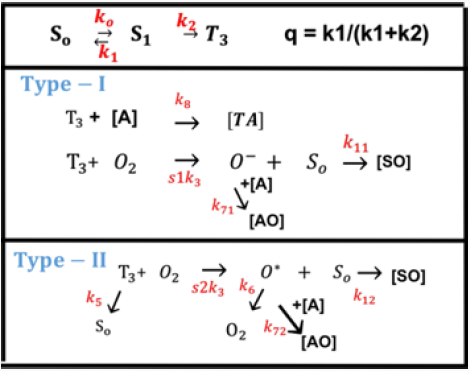
As shown in Figure 1, the photochemical kinetics has three pathways [10,11]. Defining [S0], [S1] and [T3] are the ground state, singlet excited state, and triplet excited state of PS molecules which is excited by the UV light. In type-I pathway, T3 can interact directly with the collagen substrate (A); or with the ground state oxygen (O2) to generate a superoxide anion (O-); in type-II pathway, T3 interacts with the ground oxygen (O2) to form a reactive singlet oxygen (O*) [16]. The PS excited triplet can undergo two kinds of reactions. In type I reaction, it can react directly with a substrate (cell membrane or a molecule) and transfer a proton or an electron to form a radical anion or radical action, respectively. These radicals may further react with oxygen to produce reactive oxygen species (ROS). Alternatively, in a type II reaction, the triplet RF can transfer its energy directly to molecular oxygen (triplet in the ground state), to form excited- state singlet oxygen. As show by Figure 1, both type-I and type-II reactions can occur simultaneously, and the ratio between these processes depends on the types and the concentrations of PS, substrate and oxygen, the kinetic rates involved in the process.
Figure 1: The photochemical kinetics, where [S0], [S1] and [T3] are the ground state, singlet excited state, and triplet excited state of PS molecules (see text for more details) [10].
Applications of crosslinking
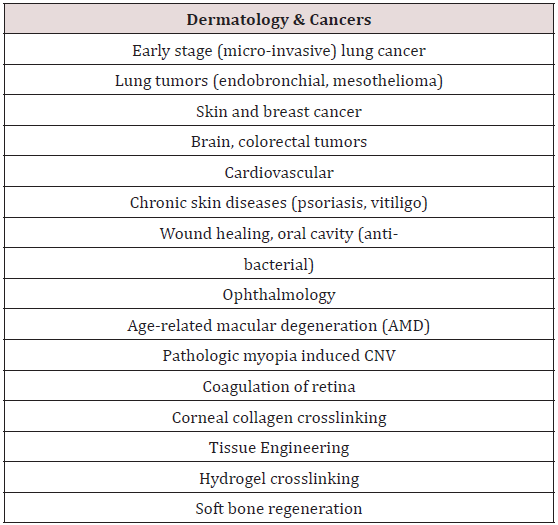
As shown in Table 2, photodynamic therapy (PDT) offers many applications in dermatology, ophthalmology and cancer treatments in various parts of human body. Photo-polymerization activated collagen crosslinking offers corneal diseases treatment and tissue engineering, such as repair of osteochondral injuries.
Tissue Engineering
Tissue engineering requires the development of a material that can support cells by providing a surface for adherence, a template for new tissue growth and a mechanical environment that can withstand the forces at a given site in the body. Chemically crosslinked collagen may be also incorporated with varied natural or synthetic polymers to create a hybrid hydrogel. Collagen hydrogels to be used for cell encapsulation or for injectable device require the following features:
a. a non-toxic catalyst or photo initiator;
b. cell-friendly light source and fast gelation times within the clinically acceptable time-scale;
c. high yields and insensitive to ambient oxygen or water;
d. a lower concentration of photo initiator or catalyst to assure good cell viability;
e. Cyto compatible hydrogel formation;
f. the precursor materials should be water- soluble for dissolution in PBS or basal media; and
g. Facile, accurate means to provide tunable mechanical and physical properties to represent ECM of muscle tissue.
The design of regenerative devices using three-dimensional (3D) scaffolds may provide the appropriate environment, mechanical support and an initial cell anchorage site for the regeneration of tissues and organs [13]. Hydrogels provide a 3D hydrated framework with tissue-like elasticity for culturing cells. They also possess the ability to encapsulate cells and molecules prior to gelation, thus affording a minimally invasive avenue to deliver cells and bioactive factors [13]. Recently, [14,15] developed a thiol-Ene reaction incorporated with collagen to form novel photo-click collagen-PEG hybrid hydrogels. The goal of a photo-click hydrogel system is to identify key reaction parameters to enable fast gelation (in seconds), minimal photo-initiator-induced toxic response, and tunable hydrogel elasticity. In the following, we will present 3 important issues: improvement of the speed and efficacy of crosslink; and the role of photosensitizer (PS) concentration, where lower PS concentration secures the cell viability, but has lower efficacy. Therefore, an optimal concentration and protocol are required.
Crosslink efficacy and safety
The crosslink efficacy is influenced by multiple factors including: the applied light intensity, exposure period and dose, the initial concentration profiles of photosensitizers (PS) and oxygen, the quantum yield of the RF triplet state, the kinetic rate constants of PS (in type-I) and oxygen (in type-II). Besides, the control of PS concentration during the light exposure are also important. Higher PS concentration provides higher efficacy, but also suffers more toxicity to the cells. Therefore, optimal ratio of C0/I0 is required for accelerated and safe crosslink. Safety issue also limits the maximum dose can be applied to the collagen, besides the limitation of PS concentration. For a type-I dominated process, the corssolink efficacy is given by Eff=1 – exp(-S) and the photoinitiation rate S-function is given by [9,10].
Where A is the effcetive absorption constant given by A=2.3mb’C0+Q; with a fit parameter m; B=aqgI (z, t); a=83.6wa’; w is the light wavelength; a’ and b’ are the molar extinction coefficient of the initiator and the photolysis product, respectively; Q is the absorption coefficient of the monomer and the polymer repeat unit. And q is the quantum yield of PS excited triplet and g is a rate constant. F(z)=1-0.5z/D is the initial PS concentration profile having a diffusion depth (D); for uniform case, D>>1 cm, and F=1. Choosing typical values of: a’=0.2(1/mM/cm), b’=0.1(1/mM/cm), Q=0.1 (1/ cm), q=0.5, aqg=0.012 cm2/mW; the mean A(z)=0.35C0F(z)+0.23, B=(0.006I0) exp(-Az), with C0 in mM, I0 in mW/cm2. Eq. (1) gives a normalized S’-function defined by S’=S1/S0, where S0=[4K/(aqg)]0.5 is a proportional constant. Based on Eq. (1), we will investigate the roles of C0, I0 and D on the spatial (z) and temporal (t) profiles of S1, for both uniform and non-uniform cases.
Figure 2: The normalized photo initiator concentration profiles for uniform (left figure) and non-uniform (right figure, with D=1.0 cm) PS distribution, for t=0 (curves 1) and t=(50, 100, 150, 200, 300, 350, 400) seconds (curves 2 to 7) with quantum yield q=1.0, a’=0.2(mM·cm)–1, b’=0.15 (mM·cm)–1, and Q=0.1 (1/cm) and I0= 10 mW/cm2.
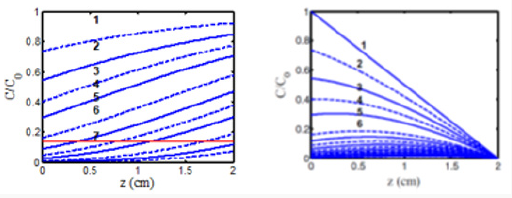
Figure 3: Time profiles of S versus exposure time (t) (left figure) and versus light dose, E, (right figure) for various light intensity I0= (10,15,20,30) mW/cm2, for D= 1.0 cm, C0=3 mM, at z=0.5 cm.
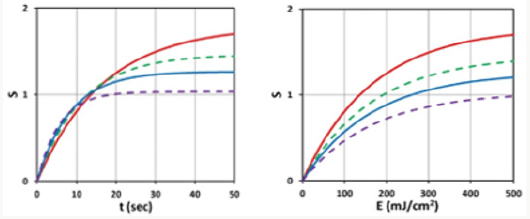
Figure 4: Spatial profiles of S for various exposure time t = (100,200,300) (for curves low to top), for light intensity I0= 10 mW/cm2, C0=2 mM for a flat distribution with F=1.
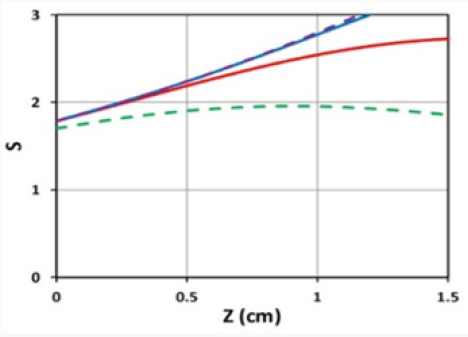
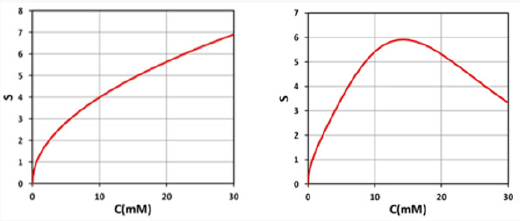
Figure 5: Shows S versus PS concentration (C0), for z=0 (left figure) and z=0.5 cm (right figure), for D=1.0 cm, I0=10 mW/cm2.
A crosslink time (Tc) may be defined by Eq. (1.b) when E1=0.87, or 0.5BTc=2, which gives us Tc=4/B= [4/(aqgI0)] exp (Az) = T0 exp (Az), with the surface crosslink time given by T0=4/(aqgI0) = 300/ I0, for aqg=0.01333. We note that this crosslink time equals to the depletion time (T*), defined by when C (z, t=T*) = C0(z)exp(-M), with M = 4, or C (z, t) is depleted to 0.018 of its initial. The crosslink time define din this study may be compared to the crossover time shown by Figure 2 of [19], indicating that Tc is a decreasing function of light intensity and the absorption constant. One may also compare the gelation kinetic profile shown by Figure 3 of [15] to the profile of the S function shown in Figure 3 of this study. Figure 2 shows the dynamical spatial profiles of the normalized photo initiator concentration for uniform (left figure) and non-uniform initial PS profile, defined by F(z) of Eq. (1.a). Figure 3 show the temporal profiles of the S-function for various light intensity, whereas its spatial profiles are shown in Figure 4. The role of PS concentration is shown by Figure 5, on the collagen surface (z=0), and at a depth of z-0.5 cm. From the above Figures 2-5, we can summarize the important features of photo-activated crosslink as follows.
a) Figure 2 shows that crosslink starts from the surface having a crosslink time (T0), and
b) volume crosslink, Tc(z), takes longer time;
c) Figure 3 (left figure) demonstrates that higher light intensity has a faster rising efficacy, but a lower steady-state value due to its faster depletion of PS concentration. As also shown by formula, Eq. (1), that the efficacy at transient state (for small dose) is proportional to tI0 0.5. However, at steady-state (with E1=1), it is a nonlinear function of [C0/I0] 0.5 or [t/E0]0.5.
d) Figure 3 (right figure) demonstrates that for the same dose (E0), all light intensity has the same crosslink time, as shown by Eq. (1.b), E1=1-exp(-Bt), with Bt=bgt=aqg (E0) which depends only on E0, independent to I0.
e) As shown by Fig. 5, higher PS concentration leads to higher S, or efficacy; and optimal PS concentration (for maximum S) exits for z>0, but not for z=0. Lower PS concentration secures the cell viability but has lower efficacy. Therefore, an optimal concentration and protocol are required.
Conclusion
Lower light intensity (I0) and higher PS concentration (C0) provides higher efficacy, but also suffers more toxicity to the cells. Higher light intensity (I0) may accelerate the crosslink but has lower efficacy (for the same dose). Therefore, optimal ratio of C0/ I0 is required for accelerated and safe crosslink. Safety issue also limits the maximum dose can be applied to the collagen, besides the limitation of PS concentration.
References
- JT Lin (1995) Critical review on refractive surgical lasers. Opt. Engineer 34(3): 668-675.
- Lin JT (2017) Progress of the 30-year laser vision technology. J Ophthalmol Clinical Res 1(1): 1-4.
- Lin JT (2016) Progress of medical lasers: Fundamentals and Applications. Medical Device Diagn Eng 1: 36-41.
- Lin JT, Chen KT, Liu HW (2017) Progress of nanotechnology for phototherapy: Fundamentals and Applications. Med Devices Diagn Eng 2: 101-107.
- JT Lin, YL Hong, CL Chang (2010) Selective cancer therapy via IR-laserexcited gold nanorods. Proc SPIE 7562: 75620R.
- Lin JT, Chen KT, HW Liu (2018) Novel techniques for improving anticancer efficacy via synergistic phototherapy. Op Acc J Bio Eng & Biosc 2(1).
- Lin JT (2017) Analysis of the efficiency of photothermal and photodynamic cancer therapy via nanogolds and photosensitizers. J Cancer Research Update 6(1): 12-18.
- Lin JT, Chen KT, HW Liu (2018) The role of drug-light dose on the efficacy of anti-cancer via oxygen-enhanced photodynamic therapy. J Cancer Research update 7: 21-23.
- Lin JT, Cheng DC (2017) Modeling the efficacy profiles of UV-light activated corneal collagen crosslinking. PloS One 12(4): e0175002
- Lin JT (2018) Efficacy S-formula and kinetics of oxygen-mediated (type-II) and non-oxygen-mediated (type-I) corneal cross-linking. Ophthalmology Research 8(1): 1-11.
- Lin JT (2018) A Critical Review on the Kinetics, Efficacy, Safety, Nonlinear Law and Optimal Protocols of Corneal Cross-linking. J Ophthalmology & Visual Neuro science 3(1): 17.
- Lin JT (2018) A proposed concentration-controlled new protocol for optimal corneal crosslinking efficacy in the anterior stroma. Invest Ophthalmol Vis Sci 59(1): 431-432.
- Chen FM, Shi S (2014) Principles of Tissue Engineering, (4th edn.), Elsevier: New York, USA.
- Holmes R, Yang XB, Dunne A, Larisa Florea, David Wood, et al. (2017) Thiol-Ene Photo-Click Collagen-PEG Hydrogels: Impact of Water-Soluble Photoinitiators on Cell Viability, Gelation Kinetics and Rheological Properties. Polymers 9: 226.
- Shih H, Liu, HY, Lin CC (2017) Improving gelation efficiency and cytocompatibility of visible light polymerized thiol-norbornene hydrogels via addition of soluble tyrosine. Biomater Sci 5(3): 589-599.
- Pereira R, Bartolo P (2014) Photopolymerizable hydrogels in regerative medicine and drug delivery. Top Biomater 6-28.
- Mazaki T, Shiozaki Y, Yamane K, Yoshida A, Nakamura M, et al. (2014) A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Scientific Report pp. 4457.
- Fairbanks BD, Schwartz, Bowman CN, Anseth KS (2009) Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials 30(35): 6702-6707.
- Anseth KS, Klok HA (2016). Click chemistry in biomaterials, nanomedicine and drug Delivery. Biomacromolecules 17(1): 1-3.
Editorial Manager:
Email:
biomedicalengineering@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...