
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4579
Case Report(ISSN: 2637-4579) 
Control of Crystal Polymorphism of CaCo3 Generated in Cracks of Cementitious Material by Change of Temperature and pH Volume 1 - Issue 5
Heesup Choi*
- Department of Civil and Environmental Engineering, Kitami Institute of Technology, Japan
Received: March 14,2018; Published: March 22,2018
Corresponding author: Heesup Choi, Department of Civil and Environmental Engineering, Kitami Institute of Technology, JAPAN, Tel: 81157269474; Email: hs-choi@mail.kitami-it.ac.jp
DOI: 10.32474/OAJBEB.2018.01.000124
Abstract
Generally, crack is inherent in reinforced concrete structures and leads to serious damage in service period. In there, recurrences of such damages will lead to the enlargement of the cracks, thereby allowing other deteriorating elements such as CO2 and Cl- to further penetrate the concrete, and this can have serious consequences for the concrete structure. On the other hand, in an environment where there is supply of water, concrete structures display "self-healing," in which some of cracks close up naturally, and this phenomenon is closely associated with the hydrates that are newly generated in the areas of crack formation.
In this study, author aimed to the kind of CaCO3 generated by self-healing phenomenon. CaCO3 is crystal polymorphism and it's reported that crystal form can be controlled by the relationship of temperature and pH. Generally, CaCO3 consist of the three kinds such as calcite, vaterite and aragonite for crystal formation. A vaterite is also generated most densely among these, and self-healing can be expected.
Therefore, an experiment is made for the purpose of establishing the condition to generate a vaterite. The supplied a saturated Ca(OH)2 solution with CO2 nano-bubble is used for the effective self-healing. Conditions of the temperature and pH are managed by 20-60°C and 8.5-12 pH. As a result, it was confirmed that the self-healing conditions of saturated Ca(OH)2 solution, which supplied CO2 nano-bubbles, displayed the most effective self-healing performance in the surface and internal sections of the cracks.
Keywords: Cementitious material; Crack; Self-healing; CaCO3; Ca(OH)2; CO2; Nano-bubble; Vaterite
Introduction
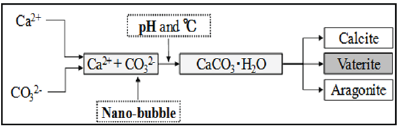
It is unavoidable that crack outbreaks in materials such as concrete. Deterioration factors penetrate into the crack, it is concern that leads to fatal damage to the concrete structure. On the other hand, there is a phenomenon called "self-healing" where the relatively small crack that occurred to the concrete under an environment with the water supply blocks up the crack [1-3]. There are three crystal forms in the crystal of CaCO3 generated by selfhealing. In this study, we focused on the CaCO3 crystal generated by self-healing and investigated better self-healing conditions by adjusting the temperature and pH, and by CO2 penetrate fine cracks as nano-ubbles. By controlling crystal polymorphism so that vaterite is formed intentionally, this could lead to the production of finer self-healing substances. Figure 1 shows the process of the self-healing of this study [4,5].
Figure 1: Process of the self-healing [2].
Materials and Methods
Using early strength Portland cement (C, density: 3.14g/cm3, Average particle diameter: 10|im), we made a specimen of water- cement ratio 40%. Cracks with average widths of about 0.15 mm were incorporated by drying for about 24 hours in a drying oven at 105°C, followed by constraining shrinkage of the cement paste to conduct experiments as a specimen "before self-healing. Table 1 shows experimental factors and experimental conditions, Table 2 shows an experimental procedure and content. The self-healing method we used is as follows. After CO2 micro/nano bubbles were provided for 4 hours, a specimen was left in a water tank of saluted Ca(OH)2 solution with added CO2 micro/nano bubbles for 20 hours. This process was counted as 1 cycle (1day).
Table 1: Experimental factors and conditions.

Table 2: Experiment procedure and contents.
Figure 2: Cracked surface after self-healing.

Results and discussion
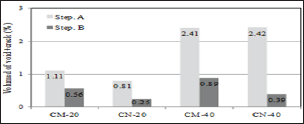
Figure 2 shows the observation results of the cracked surface part seen through the microscope. Self-healing precipitates were observed in the surface layer of cracks due to self-healing in both the CN series that supplied nano-bubbles and the CM series that supplied micro-bubbles. Figure 3 shows crack conditions at the time of cracks being introduced and after self-healing (CN-40). As long as 3D analysis images were observed, no clear change of void structure before and after self-healing was identified. Figure 4 shows the results of void volume of each case before and after selfhealing in accordance with the change of temperature condition of self-healing and supply condition of CO2. As a result, it was considered that CN series with supplied nano-bubbles were more effective than CM series with supplied micro-bubbles and also temperature condition of 40°C was more advantageous for selfhealing than that of 20°C.
Figure 3: 3D analysis image by X-ray CT.
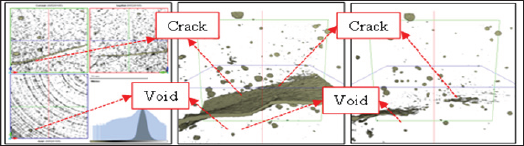
Figure 4: Volume change of (void + crack).
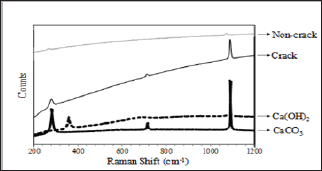
In order to identify crystalline components of precipitates generated by self-healing, we implemented Raman spectroscopy using a specimen obtained from the cracked area, on which a large amount of self-healing precipitates were adhered to (CN- 40). Figure 5 shows the result of the measurement. An extremely tiny peak wavelength of CaCO3 powder on the surface of non-crack area was identified at the same position as the peak wavelength of CaCO3 powder. Meanwhile, it was determined that, concerning cracked parts, the obvious peak wavelength corresponding to the peak wavelength of CaCO3 appeared. Moreover, it was confirmed that the peak wavelength in either case did not correspond to that of Ca(OH)2 powder. It is, therefore, considered that most of the component substance precipitated after self-healing was CaCO3.
Figure 5: Raman spectroscopy analysis.
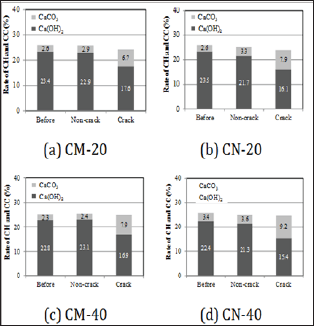
In order to chemically evaluate precipitates inside cracked parts by self-healing, we compared and evaluated the change in the amount of Ca(OH)2 and CaCO3 between before and after self-healing of individual series, using TG-DTA. Figure 6 shows the results of the analysis. In all of the series, regardless of the change of temperature condition and supply condition of carbon dioxide for self-healing, compared to Before and Non-crack, the amount of Ca(OH)2 in crack decreased, while the amount of CaCO3 in crack increased. The estimated amount of precipitates by self-healing increased in the order of CN-40 > CM-40 ≧ CN-20 > CM-20.
Figure 6: Comparison of the self-healing precipitated substances by TG-DTA.
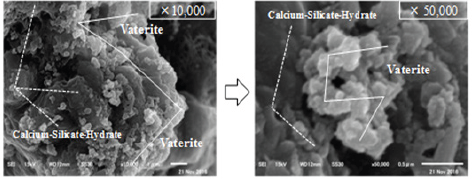
Finally, SEM analysis was carried out to confirm the shape of the crystals produced under controlled temperature and pH. Figure 7 show SEM image of cracked areas of and CN-40 with nanobubbles. As a result, almost no Ca(OH)2 was observed in cement solution under CN-40 with self-healing temperature of 40°C and pH 9.0. However, a large amount of C-S-H gel as well as a number of Vaterite, which were adhered to its' surface, were determined. For this reason, it is considered that controlling the temperature at 40°C and pH at about 9.0 makes it possible to produce self-healing precipitates of Vaterite, which is expected to contribute to more precise self-healing.
Figure 7: SEM image of cracked areas (CN-40).
Conclusion
It was confirmed that under a self-healing condition that saturated Ca(OH)2 solution with CO2 micro/nano-bubbles is supplied into cracked areas, provision of Ca2+ and CO32- facilitated CaCO3 reaction. Furthermore, it was determined that even cement material alone could demonstrate more effective selfhealing performance in comparatively large cracks of more than 0. 1mm. In addition, we could generate Vaterite that contributed to densification of cement matrix from the effect of void filling, by not only providing nano-sized super-fine bubbles that included carbonate ion into saturated Ca(OH)2 solution but also adjusting water temperature at about 40°C and pH at about 9.0 as self-healing condition.
References
- Neville, AM (1995) Properties of Concrete. Person Education Limited. London. UK: 328.
- Heesup Choi, Masumi Inoue, Risa Sengoku, Hyeonggil Choi (2017) Control of polymorphism of calcium carbonate compounds produced in cracked part of cementitious materials by self-healing. Journal of applied sciences 7(6): 1-16.
- Heesup Choi, Masumi Inoue, Sukmin Kwon, Hyeonggil Choi, Myungkwan Lim (2016) Effective Crack Control of Concrete by Self-Healing of Cementitious Composites Using Synthetic Fiber: Journal of the Materials 9(4): 1-14.
- Masakazu M (2010) Polymorph control of calcium carbonate by reactive crystallization using microbubble technique. Chemical Engineering Research and Design 88(12): 1624-1630.
- Yoshiyuki K (1994) Controls of Polymorphism and Morphology of Calcium Carbonate Compounds Formed by Crystallizing Amorphous Calcium Carbonate Hydrate. Journal of Ceramic Society of Japan 102(12): 1128-1136.
Editorial Manager:
Email:
biomedicalengineering@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...












