
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Case Report(ISSN: 2641-1687) 
Unusual Metastasis of Prostate Cancer: A Rare Case Report Volume 2 - Issue 3
Y Dkhissi1*, A Mai1, M Haloua1, B Alami1, Y Alaoui Lamrani1, M Maaroufi1, M Boubbou1, O Amrani Souhli2, M Ahsaini2, S Mellas2, JE Ammari1, MF Tazi1 and MH Farih2
- 1Radiοlοgy department of Hassan II university hospital, Sidi Mοhammed Ben Abdellah University, Fez, Morocco
- 2Urοlοgy department of Hassan II university hospital, Sidi Mοhammed Ben Abdellah University, Fez, Morocco
Received: December 13, 2019; Published: December 16, 2019
Corresponding author: Dkhissi Younes, Radiology department of Hassan II university hospital, Faculty of Medicine and Pharmacy of Fez, Sidi Mohammed Ben Abdellah University, Fez, Morocco
DOI: 10.32474/JUNS.2019.02.000140
Abstract
Prostate cancer is the second death causing men cancer after lung cancer [1]. It is the most common men cancer over the age of 50. Metastasis for this type of cancer are mainly lymph node and bone. Visceral and cerebral metastasis are unusual, rare and terminal, reported in less than 4% of cases and often postmortem. [2] We report the case of a patient suffering from prostate adenocarcinoma with a high-risk according to the AMICO classification, who has been operated (open prostatectomy without preservation of neurovascular bundle). The patient presented two years after radical treatment a biological recurrence and started complaining headache and visual problems that led to the discovery of dural metastasis.
Keywords: Cancer; Prostate; Dural metastasis
Introduction
Prostate cancer is the most common men cancer over 50 years. It represents the first urological cancer and the second after lung cancer [3,4]. It is a global disease whose incidence increases with age and varies by races and regions [3,5]. The diagnosis is based on digital rectal exam and an abnormal increase of prostate specific antigen (PSA), which may envisage and predict tumor extension (PSA>100ng/ml) [5,6]. The anatomy pathology study confirms the diagnosis and specifies the type and grade of histology. Prostatic cancers are dominated in 95% by adenocarcinomas [7]. Less than 2% of patients develop brain metastasis. Autopsy series have certified the rarity of brain secondary locations [8]. The most common metastasis are lymph node and bone (reaching 40% of patients at the time of diagnosis and 86% at the terminal stage). Visceral and brain metastasis are rare and terminal [2].
Patient and Methods
54-year-old patient with history of operated shoulder fracture 9 years ago, presented low urinary tract symptoms (poor steam, sensation of incomplete bladder emptying) associated to approximately eight kilograms of weight loss. The initial clinical examination objectified an enlarged asymmetrical prostate (60g) with areas of firmness and induration. The PSA level was 18 ng/ml. A trans rectal ultrasound was performed and showed an increased prostate volume with diffuse hypo echogenic areas. The kidneys were normal, without dilatation. The prostatic biopsy revealed a moderately differentiated prostatic adenocarcinoma with a Gleason score of 7 (4+3). This ranks the patient as a highrisk patient according to the AMICO classification. The initial extension CT didn’t objectify any remote secondary locations. The patient was operated (open prostatectomy without preservation of neurovascular bundle). The patient presented a good clinical, biological and radiological response. Two years later, the patient had a biological recurrence with a high PSA level superior to 0.2 μg/L, he was programmed for radiotherapy sessions. Meanwhile, the patient experienced headaches with a decrease in visual acuity. A cerebral CT scan was performed with evidence of dural and skull metastasis (Figures 1 & 2).
Figure 1: Axial contrast enhanced cerebral CT (A,A1,A2) with sagittal reconstructions (B,B1) : Thickness and enhancement of the dura of the convexity and the base respecting the dura of the falx cerebri and tentorium cerebelli (Red Arrows).
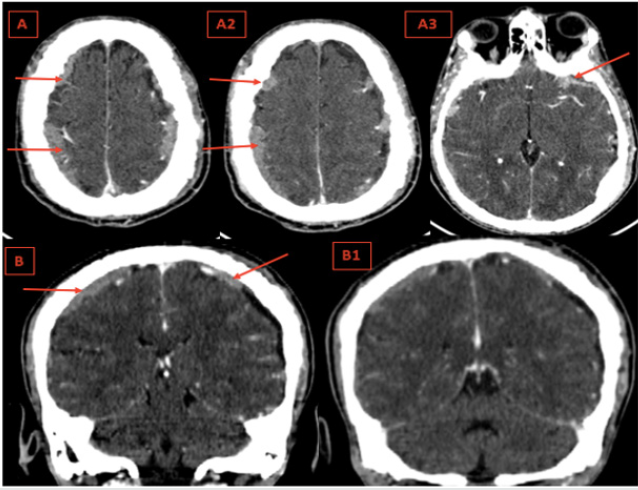
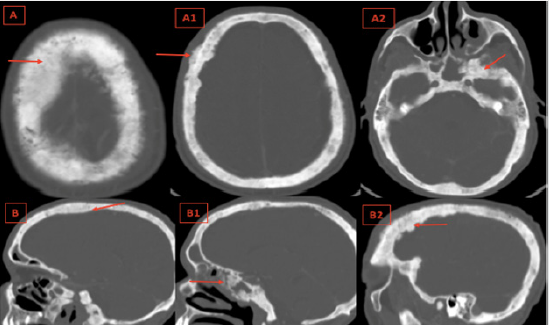
Figure 2: Axial Cerebral bone window CT (A,A1,A2) with sagittal reconstructions (B,B1,B2): Diffuse osteoblastic lesions of the cranial vault and the skull base, irregular bone thickening and loss of differentiation between the inner, outer tables and the spongy bone of the diploé. (Red Arrows).
Discussion
The pathogenesis of dural metastasis is not yet elucidated
[8]. There are two main routes of dissemination: Hematogenous
diffusion from a cerebral localization or from the cervical lymph
nodes through the cranial foramina [9,10]. Theoretically, the cancer
cell detaches itself from the primary tumor and crosses several
barriers to reach the target organ which is the dura [11]. Prostate
cancer presents a great tropism for dura [12]. It is responsible of
9% of dural metastasis and comes behind breast cancers 32% and
melanomas 11% [13].
Diffuse dural metastatic localization are exceptional and
associated with a diffuse metastatic infiltration of the cranial vault
and skull base; these localizations are observed in prostate cancer;
bone metastatic involvement is represented by diffuse osteoblastic
lesions of the skull base, bone thickening and loss of differentiation
between the inner, outer tables and the spongy bone of the diploé.
The dura being in contact with the infiltrated cranial vault and skull
base appears thickened and enhanced after injection of the contrast
agent, focal thickening is possible and can simulate meningioma;
subcutaneous tissues can also be infiltrated; the falx cerebri and
tentorium cerebelli are preserved. [14]
A diffuse thickening of the dura of the convexity and possibly
of the base respecting the dura of the falx cerebri and tentorium
cerebelli evokes diffuse tumor infiltration, most often related to
metastatic infiltration of cranial vault and skull base, essentially in prostate cancer. [14] Many autopsy studies affirmed the rarity
of the brain metastasis emanating from prostate cancer [15]. In
a large postmortem study about metastatic patterns of prostate
cancer, Saitoh et al. [16], noted no cases of brain metastasis.
In our case, the brain metastasis was diagnosed while the
patient was not on any treatment including chemotherapy or
hormone therapy. It was revealed 24 months later from the initial
radical treatment. This result approaches the result obtained by MD
Anderson’s experience with brain metastasis in prostate cancer,
published in 1999 by Mccutcheon and his partners that revealed
that the average delay between the diagnosis of prostate cancer
and the first discovery of brain metastasis was approximately 28
months. [17] Another enlarged and updated study realized in 2003
by Tremont-Lukats and partners revealed that the brain metastases
represented only 0.6%. [18]
The median survival of cancer prostate patient with central
nervous system relapse in clinical series is poor [8].In the MD
Anderson series, the median survival was 1 month, the patients
receiving palliative radiotherapy showed an an improved median
survival of 3.5 months (2.4 to 4.6 months). In our case, the patient
is still alive after starting palliative radiotherapy for 5 months [19].
Conclusion
Brain metastasis of prostate cancer are rare, often discovered in postmortem. It requires close monitoring; the rise of PSA levels should alarm. Imaging is important to confirm the diagnosis and to eliminate the other etiologies. The factors underlying the selective tropism of the prostatic malignancy for the brain remain to be defined.
References
- Steg A, Flam T, Zerbib M, Desligneres S, Eschwege F, et al. (1991) Cancer de la prostate in: Cancers urogénitaux: Steg A. Eschwege F Paris Flammarion Médecine Sciences, pp: 230-307.
- Ameur A, Touiti D, EL Mostarchid B, EL Alami M, Jira H, et al. (2001)Métastasescérébralesd’un cancer de la prostate: régression sous traitement hormonal. Prog Urol, 11: pp 1298-301.
- Droz JP, Flechon A, Terret C (2003) Cancer métastatique de la prostate. Rev, 53: pp 2258-2262.
- Herr HW, Kornblith AB, Ofman U (1993) Comparison of the Quality of Life of patients with metastatic prostate cancer who received or did not receive hormonal therapy. CANCER, 71(3 suppl): 1143-1150.
- Conti G, La Torre G, Cicalese V, Michiletti G, Ludovico MG, et al. (2008) Prostate cancer metas- tases to bone: observational study for the evaluation of clinical presentation, course and treatment patterns. Presentation of the Metauro protocol and of patient baseline features. Arch Ital Urol Androl, 80(2): 59-64.
- Chartier E (1998) Cancer de la prostate. Urologie Ed,Estem et Medline, paris, pp. 119-134.
- Birtle AJ, Freeman A, Masters JRW, Payne HA, Harland SJ (2003) Clinical features of patients who present with metastatic pros- tate carcinoma and serum Prostate Speci c Antigen (PSA) levels < 10 ng/ml: The “PSA negative” patients. Cancer, 98(11): 2362-2367.
- Craig J, J Woulfe MD, J Sinclair MD, S Malone MD (2015) Isolated brain metastasis as first site of recurrence in prostate cancer: case report and review of the literature. Current Oncology, Vol. 22(6).
- Destrieux C, Becker H, Jan M (1995)Métastasesintracrâniennes in: Neurochirurgie. Ellipses, 145-152.
- Hoang-Xuan K, Napolitano M, Cornu P, Delattre JY (1999)Métastasescérébrales et leptoméningées des cancers solides. EncyclMé
- Kehrli P (1999)Biologie des métastasescéréNeurochirurgie, 45: 364-368.
- Takakura K, Sano K, Hojo S (1982) Metastatic tumors of the central nervous system. Tokyo: IgakuShoin.
- Kehrli P (1999)Épidémiologie des métastasescéréNeurochirurgie, 45: 357-363.
- Dietemann JL, Correia Bernardo R, Bogorin A, Abu Eid M, M Koob, et al. (2005) Les prises de contrasteméningéesnormales et pathologiquesen IRM. Journal de radiologie, Vol 86(11): 1659-1683.
- Baumann MA, Holoye PY, Choi H (1984) Adenocarcinoma of prostate presenting as brain metastasis. Cancer, 54: 1723-1725.
- Saitoh H, Hida M, Shimbo T, Nakamura K, Yamagata J, et al. (1984) Metastatic patterns of prostatic cancer. Correlation between sites and number of organs involved. Cancer, 54(12):3078-3084.
- McCutcheon IE, Eng DY, Logothetis CJ (1999) Brain metastasis from prostate carcinoma: antemortem recognition and outcome after treatment. Cancer;86(11):2301-2311.
- Tremont-Lukats IW, Bobustuc G, Lagos GK, Lolas K, Kyritsis AP, et al. (2003) Brain metastasis from prostate carci-noma: the M.D. Anderson Cancer Center experience. Cancer, 98(2):363-368.
- N'Dri Oka D, Varlet G, Boni N, Broalet E, Boukassa L, et al. (2000) Métastaseduraled'un adénocarcinome de la prostate simulant un hématome sous-dural aiguintracrâ Journal of Neuroradiology, Vol 27(4): 282.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




