Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Review Article(ISSN: 2641-1687) 
The Clinical Impact of Hexylaminolevulinate-Blue Light Cystoscopy on Non-Muscle Invasive Bladder Cancer (NMIBC) Recurrence and Progression: A Critical Review Volume 1 - Issue 5
Omer Abdalla1,2,3*, Seshadri Sriprasad1,2 and Sanjeev Madaan1,2
- 1Dartford and Gravesham NHS Foundation Trust
- 2Canterbury Christ Church University
- 3Portsmouth Hospitals NHS Foundation Trust
Received: May 14, 2019; Published: May 22, 2019
Corresponding author: Omer Abdalla, Dartford and Gravesham NHS Foundation Trust, Canterbury Christ Church University, Portsmouth Hospitals NHS Foundation Trust, UK
DOI: 10.32474/JUNS.2019.01.000123
Abstract
The superficial bladder cancer is associated with high risk of recurrence and progression, necessitating lengthy follow up and repeated endoscopic treatments. White light cystoscopy (WLC) is the current gold standard for the diagnosis, treatment and surveillance, however the sensitivity and specificity isn’t perfect with risk of missing some tumours. A photodynamic diagnosis (PDD) using Blue-light cystoscopy is a novel concept proposed to overcome the WLC shortcomings. This critical review synthesizes the evidences on the effectiveness of Blue light cystoscopy (BLC) for the diagnosis of non-muscle invasive bladder cancer (NMIBC), and the impact of photodynamic diagnosis on the recurrence rate and progression of superficial bladder cancer.
Introduction
Bladder cancer (BC) is considered as the second most common urological malignancy [1] and the eighth most prevalent cancer in males and 14th most common malignancy in females [2]. In 2014 It was estimated that around 10,000 new BC cases were diagnosed in UK and accounted for 5,400 deaths which represents around 3% of all cancer death in UK [2]. The cost for bladder cancer diagnosis, treatment, and follow up for 5 years was £55.39 million, 63.6% (£35.25 million) of this amount was the cost for superficial bladder cancer. The mean cost per patient was higher for bladder cancer than for prostate cancer (£8349 vs. £7294) [3]. Non-muscle invasive bladder cancer (NMIBC) accounts for 75% of bladder cancer cases [3]. NMIBC includes Papillary lesions confined to bladder urothelium (Ta) or submucosa (T1) and Carcinoma in situ (CIS) [4,5]. NMIBC is associated with significant rate of recurrence up to 61% within 1 year and 78% within 5 years of resection, and moderate risk of progression to muscle invasive lesion in up to 17% at 1 year and up to 45% at 5 years [6]. This high percentage of recurrence and progression may be attributed to a number of factors such as; misdiagnosis of bladder cancer (false negative results), incomplete transurethral resection of the bladder tumour (TURBT) with resultant residual lesions or overlooked CIS lesions [7]. Therefore, robust diagnosis and lengthy follow up is of paramount importance, together with treatment costs it makes NMIBC the most costly neoplasm [8]. White light cystoscopy (WLC) remains the gold standard for superficial bladder cancer diagnosis, surveillance and follow up [4,9,10]. However, the sensitivity and specificity of WLC is not perfect particularly for CIS lesions, it was estimated that around 3.4% to 20.6 % of single lesions and 7.4% to 45.8% of multiple tumours are still overlooked by conventional WLC [9,11]. CIS has been estimated to have high risk 40-80% towards progression into muscle invasive cancer if not treated [12]. Therefore, improving diagnosis and adequate tumour resection (TURBT) cannot be overemphasized. Photodynamic diagnosis (PDD) by way of blue-light cystoscopy (BLC) is one of the new novel diagnostic tools which help enhancing NMIBC diagnosis, thus, reduce the recurrence and progression and overcome the drawbacks of the conventional cystoscopy.
Methodology
This is a non-systematic critical review of the literature focusing on the clinical impact of BLC. The literature search was conducted on Medline in May 2018 using the following MeSH terms: Blue light cystoscopy, Photodynamic cystoscopy, Photodynamic diagnosis, Fluorescence cystoscopy, Fluorescence-guided, and hexylaminolevulinate (HAL) which is the only approved pro-drug for use in USA and Europe. The search yielded 92 publications. The inclusion criteria were limited to the publications in Englishlanguage, literature of the last 10 years, studies using HAL only, and the relevant studies that reported the impact on recurrence and progression. The expert’s opinions, article reviews, irrelevant articles, and the studies not using HAL were excluded.
The PDD mechanism and technique
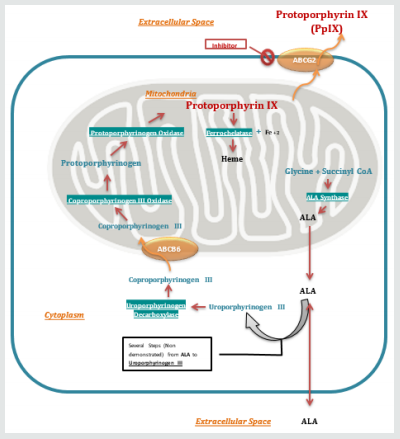
PDD depends on photoactive properties of 5-aminolevulinic acid (5-ALA) or its hexyl ester Hexylminolevulinate (HAL), both lead to excessive accumulation of photosensitive molecule Protoporphyrin IX (PpIX) preferentially in the cancerous and pre-neoplastic cells. Malignant cells with high Pp IX emit redfluorescence when exposed to blue light with specific wavelength (375-445nM) [13-15]. After intravesical instillation of 5-ALA or HAL, the drug is then absorbed into urothelial cells and incorporated in the Haem-biosynthesis pathway [14]. Lack of the negative feedback of the Haem biosynthesis metabolism in cancerous cells results in excessive accumulation of Pp IX (Figure 1) [13,14]. Two pro-drugs have been studied in Photo dynamic diagnosis: 5-ALA and HAL. 5-ALA has limited cellular penetration making it less effective. HAL is superior to 5-ALA due to its lipophilic property which allows higher tissue uptake, moreover it induces excessive accumulation of Pp IX, therefore, gives remarkable fluorescence at low concentrations as opposed to 5-ALA, and a shorter instillation time (0.5hr) [13,16]. In most studies the instillation time of 1 hour has been used. HAL is the only agent approved for clinical use in USA and Europe [17]. (Figure 1).
PDD Technique: 50mls of HAL solution prepared and instilled into bladder after complete emptying by way of catheter. Patients keep solution for 1 hour and then void or the bladder is drained in theatre prior to Blue-light cystoscopy examination.
NMIBC Detection by BLC
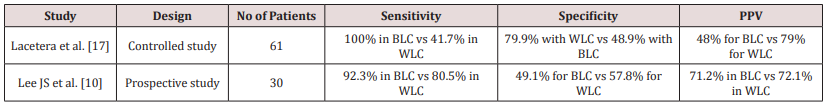
Several studies and meta-analysis studies have showed that Blue-light cystoscopy is better than conventional cystoscopy in detecting NMIBCs. In a multicentre randomized controlled trial (RCT) involving 814 patients with suspected or recurrent NMIBC undergoing either conventional cystoscopy guided or blue light cystoscopy guided TURBT, blue light cystoscopy detected 16% further lesions that were not detected by conventional cystoscopy (p= 0.001) [18]. This was particularly evident with regard to CIS detection as 32% of patients who had CIS lesions, their lesions were only visible with blue light cystoscopy (p<0.0001). Moreover, there was no significant difference in the false positive results between both groups (11% in BLC vs 10% in WLC). A recent RCT conducted by Karaolides et al. [19], involved 102 patients showed that the detection rates in BLC arm were 99.1% compared with74.5% of the white-light cystoscopy [19]. In a prospective, comparative, controlled study including 61 patients with suspected (n=15) or known bladder cancer (n= 46), the overall sensitivity of BLC was 100% as opposed to WLC 41.7%, while the specificity of WLC and BLC was 79.9% and 48.9% respectively. The positive predictive value of BLC was 48% (95% CI: 0.447-0.523) compared to 79% (95% CI: 0.856-0.734) of the WLC (Table 1). Sensitivity of BLC for CIS and High grade NMIBC was 100 %, while it was 34.1% (for High grade NMIBC) and 39% (for CIS) of the WLC [17]. Lee JS et al. [10], conducted prospective study in which 30 patients with confirmed bladder cancer on WLC, the sensitivity of BLC was 92.3% versus 80.5% of the WLC (p= 0.021), while specificity was 49.1% for BLC compared to 57.8% for WLC (statistically insignificant). The positive predictive value of BLC was 71.2% versus 72.1% of WLC, while negative predictive value was 81.8% of BLC compared to 68.8% of WLC (Table 1) [10]. Sfetsas et al. [9] showed that BLC detected additional CIS lesions in 22 patients which were otherwise undetectable by WLC (9) (Table 1). The meta-analysis by Burger et al. [20] looked into six studies (using HAL-BLC for PDD) which showed statistically significant detection rate of the BLC for Ta lesions. In all studies CIS detection rate by BLC was between 31.9% and 70.6% of all CIS lesions detection (p < 0.001; OR 12.372; 95% CI, 6.343-24.133), overall 40.8% additional CIS and 10.8% additional T1 lesions were identified by BLC alone (p=0.050; OR: 2.253; 95 % CI, 0.999-5.081) which means superiority of BLC detection in Ta,T1, and CIS [20]. Likewise, the meta-analysis of Di Stasi SM et al. [16] (2015), showed superiority of BLC detection of Ta, T1 and CIS lesions in six studies [16]. It is apparent that BLC has improved rate of tumours detection, especially for CIS which carries high risk of progression into muscle-invasive carcinoma. Therefore, improved bladder tumour detection using BLC may have an impact on the recurrence rate and progression of NMIBC.
Impact on NMIBC recurrence
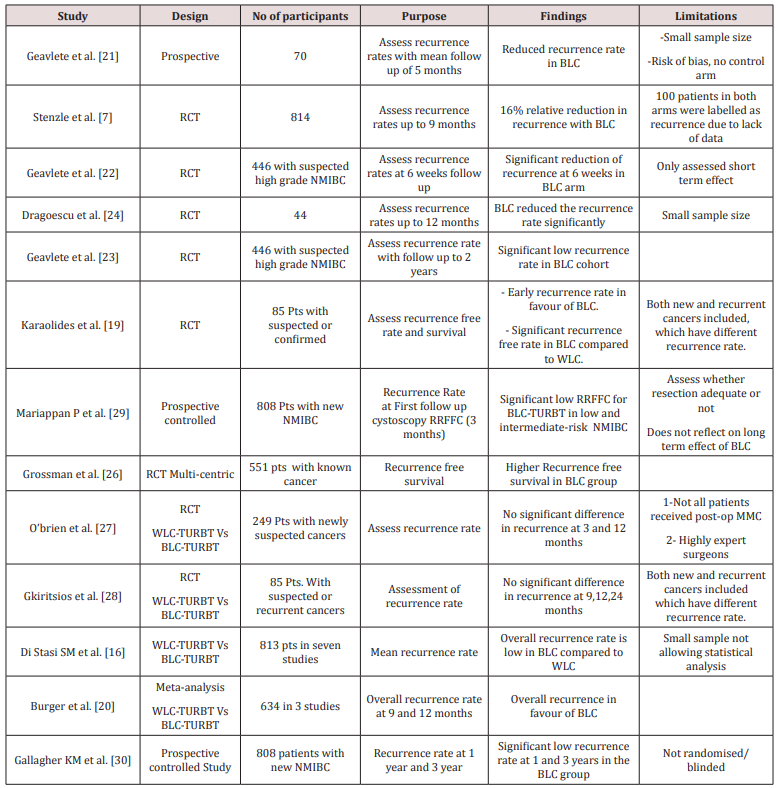
Numerous studies have investigated the impact of BLC on the recurrence rate of the bladder cancer, which includes RCTs and meta-analysis. A study by Geavlete et al. [21] in which 70 consecutive patients underwent WLC guided TURBT for suspicious lesions, followed by BLC guided resection of the fluorescent lesions in the same patients, this showed significant reduced recurrence rate at 5-months follow up of 6.4% vs 24.2% in favour of BLC. However in this study the sample size was small, it was not randomized, using bladder maps for lesions detected and subsequently resected under guidance of both scopes on the same patients might have led to bias in assessing the recurrence accurately since authors did not describe how did they attribute each recurrence to the relevant groups, by using same patients to represent the control and study groups [21]. A randomized controlled study by Stenzle et al. [18] recruited 814 patients, who underwent TURBT either by WLC or BLC and then followed up with conventional cystoscopy at 3, 6, and 9 months. The recurrence rate was 47% in BLC group versus 56% in WLC with relative reduction of 16% for the tumour recurrence. However, about total of 100 patients in both groups were labelled as having recurrence due to lack of histology results and absence of follow up data at 9 months [18] Geavlete et al. [22] investigated the impact of BLC on recurrence at short term and reported the results of randomized blinded controlled trial, in which 446 patient with suspected high grade NMIBC underwent white light cystoscopy guided TURBT followed by Blue light cystoscopy resection or diathermy in one group, and only WLC-TURBT in the second group, then recurrence rate was assessed at 6 weeks during the re-TURBT. The recurrence rates were statistically significant for CIS (4.3% vs 27.8%) and pT1(15.4% vs 35.1%) in favour of blue light cystoscopy group, while this was not significant for pTa G3 10% in BLC group compared 22.2% in WLC group probably due to small number of patients with this grade (19 in both groups). The overall recurrence rate in patients with high grade NMIBC was 17.2% in BLC group compared to 37% in WLC group [22]. The same authors reported the results of the 2-year follow up of this RCT, and the difference in recurrence rate was significant in favour of BLC-arm 0.8% vs 6.1% at 3-month, 21.6% vs 32.5% at 1-year, and 31.2% vs 45.6% at 2-year (p= 0.001)[23]. A randomized controlled study reported by Dragoescu et al. [24] in which 44 patients were randomized into BLC arm and WLC arm and followed up to 12 months, this showed that BLC resulted in reduced recurrence rate by 9.1% at 3-month, 13.6%, 22.7%, and 27.3% at 6, 9 and 12 months respectively, and deemed statistically significant (p=0,0461) [24].However, the sample size was small in this study. In prospective, randomized open label study by Hermann et al. [25] in which 233 patients with suspected new or recurrent NMIBC were randomized to white light guided TURBT (WLC-TURBT) or WLC-TURBT followed immediately by Blue light cystoscopy guided TURBT (BLC-TURBT). The recurrence rate was significantly low 30.5% in BLC-TURBT group compared to 47.3% in WLC-TURBT (p= 0.05) followed up to 12 months, with significant longer recurrence-free period in BLC-TURBT group (P=0.02) [25]. The RCT by Karaolides et al. [19], where 102 patients were randomized consecutively into two groups and first group underwent WLC-TURBT (n=51) while the second one underwent BLC-TURBT (n=51) followed by intravesical instillation of Epirubicin to all patients. The median follow-up was 14 months (range 4.5-25) in the WLC group and 17.5 months (range 6-25) in the BLC group. Early recurrence at 3 months was (2.4%) of the BLC group compared with 13.3% of the WLC group (P =0.001). The recurrence-free rates (RFS) of patients in the WLC group at 12 and 18 months were 56.3% and 50.6%, respectively, as opposed to 91% and 82.5% for patients in BLC group (log-rank test P 0.0006). At 12 and 18 months of follow-up, the BLC group had a favourable RFS compared with the WLC group when multifocal tumours (89.7% vs 27.1%; 89.7% vs 13.6%; log-rank test P.001), but there was no significant difference in RFS between the two groups when single lesions were resected [19]. However, this study included both new and recurrent bladder cancer patients which might have different recurrence potentials. The multi-centric RCT by Grossman et al. [26] included 551 patients with known bladder cancer assessed recurrence free survival in long term follow up (up to 55 months). It was estimated that the median recurrence free survival was 9.6 months in the white light group and 16.4 months in the Blue-light cystoscopy group (p = 0.04) [26]. In the RCT by Obrien et al. [27], 249 patients with suspected bladder cancer were recruited and underwent WLC guided TURBT Vs BLC guided TURBT followed by single-dose Mitomycin (MMC). Total 189 had NMIBC and the recurrence rate was 20% in BLC-TURBT compared to 17% of the WLC-TURBT at 3 months (p=0.7), while it was 16% in BLC-TURBT versus 22% in WLC-TURBT at 12 months (p=0.4) therefore it was deemed not statistically significant [27]. The drawbacks of this study are; 1) MMC instillation was not performed in all patients, 61/97 in BLC group compared to 68/88 (p= 0.04), 2) low recurrence rate in the control was presumably due to high quality resection and experience of surgeons in performing BLC made them better performers with WLC, as it was performed by experienced Bladder cancer surgeons. Therefore, it cannot be generalized to all UK centres where most of TURBTs are performed by trainees or general Urologists. Gkiritsios et al. [28] reported the results of the RCT which recruited 85 patients with suspected or confirmed and recurrent NMIBC randomized into two groups; one underwent BLC guided TURBT after WLC, and second group underwent WLC guided resection. It did not find any significant difference in the recurrence rate between the two groups [28]. However, this study recruited newly suspected and recurrent cancers, each of which has different recurrence rate, and the study did not report whether the two groups have comparable numbers of new and recurrent NMIBC. A prospective non-randomised controlled (observational) study included 808 patients, in which one cohort underwent WLC guided good quality TURBT (n=438) and the other group underwent BLC guided good quality TURBT (n=370) then a recurrence risk at first follow up cystoscopy (RRFFC) was measured. RRFFC (including residual lesion at early re-TURBT in high risk NMIBC) was 13.6% for BLC-TURBT versus 30.9% WLC-TURBT (95% CI =1.6-5.0, OR=2.9; P< .001). RRFFC was significantly low after BLC-TURBT for low and intermediate risk NMIBC [29]. A follow up study to this study was carried out by Gallagher KM et al. [30] and the recurrence rate was significantly low at 1 and 3 years in BLC group, 39.0% in BLC vs 53.3% in WLC (OR = 0.56 (0.35–0.90) p = 0.02) [30]. The RCT by Dragoescu et al. [31] in which 113 patients were randomized to BLC group and WLC group and all patients received post-TURBT chemotherapy and followed up to 5 years. The overall 5-year recurrence rate was significantly lower in the BLC-arm 49.1% versus 67.9% in WLC-arm with 19% reduction in recurrence when using BLC for resections [31]. The meta-analysis by Di Stasi SM et al. [16] measured the mean recurrence rate in seven studies and found that the recurrence rate is 14.8% in BLC-TURBT as opposed to 32.2% of the WLC-TURBT at 12 months follow up [16]. Another meta-analysis by Burger et al. [20] which assessed parallel groups in 3 studies showed significant lower overall recurrence rate in favour of BLC (34.5%) compared to WLC group (45.4%). The recurrence was markedly lower for High and low risk tumours in the BLC group (p = 0.050 and p= 0.029, respectively) but no significant difference in the intermediate risk patients between both BLC and WLC [20] (Table 2).
Impact of Blue Light Cystoscopy on Progression
The international Bladder Cancer Group (IBCG) has recently proposed a new standard definition for bladder cancer progression which includes any one of: an upgrade in T stage from Ta to T1 or CIS, CIS to T1, progression to T2 or T3 or T4, lymph node involvement, distant metastasis or upgrading from low grade to high grade [32]. Based on this definition Kamat et al. [33] carried out a reanalysis of a multicentre RCT with extended follow up (up to 4.5 years), this showed a lower rate trends of progression when using BLC, though no significant statistical difference between WLC and BLC. Same analysis found a significant longer time to progression in favour of BLC group (p= 0.05) [33]. In prospective controlled study with long term follow up (up to 3 years) by Gallagher KM et al. [30] the progression to high grade and muscle invasive bladder cancer (MIBC) was significantly lower in BLC group 5.5% versus 13.3% in WLC group (p = 0.03) but progression into MIBC alone was not significant between both groups [30]. In RCT reported by Geavlete et al. [23] there was no difference between WLC-arm and BLC-arm in the progression rates 2.4% vs 4.4% at 1-year (p= 0.195) and 4% vs 7% at 2-year (p= 0.123), however in this study BLC has led significantly to change in recurrence - and progression-risk subgroups with resultant change in post-TURBT treatment (BCG and MMC) when compared to WLC (19% vs 6.3%)(23).
Cost Impact of BLC
Klaassen et al. [34] assessed the cost of BLC guided TURBT versus WLC guided TURBT in three provinces in Canada showed reduced the 5-year amortized cost to 1295- $1372/patient when using BLC, this reflects cost-saving as a result of reduction in number of recurrences. Furthermore, reduction in the recurrence rates would translate into decreased number of beds needed for TURBTs and this was estimated to save $842 per bed/day in Ontario [34]. Garfield et al. [35] showed cost saving of 15% ($4660/patient) over 5 years when using BLC for initial TURBT [35].
Limitations of BLC
Photodynamic diagnosis has few drawbacks such as high false positive rates for non-malignant lesions like dysplasia, reactive atypia, and denuding cystitis with resultant overtreatment [9]. Furthermore, false positive fluorescence can result from inspection of urothelium at an acute angle, commonly when looking at bladder neck, trigone or diverticula, since the mucosal cells overlap on each other resulting in false high PpIX concentration in normal cells [28]. Also, the practical obstacles of ensuring that patients have the HAL instillation and then emptying within the recommended durations prior to TURBT may render this procedure at least time and labour consuming if not less-favourable.
Conclusion
Blue-light cystoscopy is superior to conventional cystoscopy in detecting NMIBC and has positive impact on recurrence; however, it does not have a significant impact on the progression risk which might be due to the nature of the bladder cancer itself. Photodynamic diagnosis increases the recurrence free survival and impact on patient’s general wellness. The results of randomized controlled trial phase III (PHOTO trial, ISRCTN84013636) assessing the clinical impact and cost-effectiveness of blue-light cystoscopy guided TURBT in UK healthcare model is awaited and will provide crucial answers as to whether this procedure is worthwhile for the National Health Service (NHS).
References
- Reynard J, Brewster S, Biers S Oxford handbook of urology. (3rd edn), Oxford University Press, Oxford, UK, pp. 246.
- (2018) Cancer research UK, Bladder cancer statistics for the UK.
- Sangar VK, Ragavan N, Matanhelia SS (2005) The economic consequences of prostate and bladder cancer in the UK. BJU Int 95(1): 59-63.
- Babjuk M, Böhle A, Burger M, Capoun O (2017) EAU Guidelines on Non–Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 71(3): 447-461.
- Hall MC, Chang SS, Dalbagni G, Pruthi RS (2007) Guideline for the Management of Nonmuscle Invasive Bladder Cancer (Stages Ta, T1, and Tis): 2007 Update. J Urol 178(6): 2314–2330.
- Sylvester RJ, Van Der Meijden APM (2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49(3): 466–475.
- Kausch I, Sommerauer M, Montorsi F, Stenzl A (2010) Photodynamic Diagnosis in Non-Muscle-Invasive Bladder Cancer: A Systematic Review and Cumulative Analysis of Prospective Studies. European Urology 57(4): 595-606.
- Svatek RS, Hollenbeck BK, Holmäng S (2014) The economics of bladder cancer: Costs and considerations of caring for this disease. Eur Urol 66(2): 253-262.
- Sfetsas K, Mitropoulos D (2016) Reducing understaging of bladder cancer with the aid of photodynamic cystoscopy. J Egypt Natl Canc Inst 28(2): 89–94.
- Lee JS, Lee SY, Kim WJ, Seo S (2012) Efficacy and safety of hexaminolevulinate fluorescence cystoscopy in the diagnosis of bladder cancer. Korean J Urol 53(12): 821–825.
- Brausi M, Collette L, Kurth K, Van Der Meijden AP (2002) Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: A combined analysis of seven EORTC studies. Eur Urol 41(5): 523–531.
- Cheng L, Cheville JC, Neumann RM (1999) Survival of patients with carcinoma in situ of the urinary bladder. Cancer 85(11): 2469–2474.
- Frampton JE, Plosker GL (2006) Hexyl aminolevulinate: In the detection of bladder cancer. Drugs 66(4): 579–580.
- Oude Elferink P, Witjes JA (2014) Blue-light cystoscopy in the evaluation of non-muscle-invasive bladder cancer. Ther Adv Urol 6(1): 25–33.
- Inoue K, Matsuyama H, Fujimoto K (2016) The clinical trial on the safety and effectiveness of the photodynamic diagnosis of non-muscle-invasive bladder cancer using fluorescent light-guided cystoscopy after oral administration of 5-aminolevulinic acid (5-ALA). Photodiagnosis Photodyn Ther 13: 91-96.
- Di Stasi SM, De Carlo F, Pagliarulo V (2015) Hexaminolevulinate hydrochloride in the detection of nonmuscle invasive cancer of the bladder. Ther Adv Urol 7(6): 339-350.
- Lacetera V, Cantoro U, Montesi L, Cantoro D (2017) Blue light cystoscopy with hexylaminolevulinate: Our 7 years’ experience. Arch Ital di Urol e Androl 89(1): 39–41.
- Stenzl A, Burger M, Fradet Y, Mynderse LA (2010) Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol 184(5): 1907–1913.
- Karaolides T, Skolarikos A, Bourdoumis A, Konandreas A (2012) Hexaminolevulinate-induced fluorescence versus white light during transurethral resection of noninvasive bladder tumor: Does it reduce recurrences? Urology 80(2): 354-359.
- Burger M, Grossman HB, Droller M, Schmidbauer J (2013) Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: A meta-analysis of detection and recurrence based on raw data. Eur Urol 64(5): 846–854.
- Geavlete B, Multescu R, Georgescu D, Jecu M, Geavlete P (2009) HAL fluorescence cystoscopy and TURB one year of Romanian experience. J Med Life 2(2): 185–190.
- Geavlete B, Jecu M, Multescu R, Georgescu D (2010) HAL blue-light cystoscopy in high-risk nonmuscle-invasive bladder cancer-Re-TURBT recurrence rates in a prospective, randomized study. Urology 76(3): 664–669.
- Geavlete B, Multescu R, Georgescu D, Jecu M (2012) Treatment changes and long-term recurrence rates after hexaminolevulinate (HAL) fluorescence cystoscopy: Does it really make a difference in patients with non-muscle-invasive bladder cancer (NMIBC)? BJU Int 109(4): 549–556.
- Drăgoescu O, Tomescu P, Pănuş a, Enache M (2011) Photodynamic diagnosis of non-muscle invasive bladder cancer using hexaminolevulinic acid. Rom J Morphol Embryol 52(1): 123–127.
- Hermann GG, Mogensen K, Carlsson S, Marcussen N (2011) Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in T a/T1 patients: A randomized two-centre study. BJU Int 108(8 B).
- Grossman HB, Stenzl A, Fradet Y, Mynderse LA (2012) Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. J Urol 188(1): 58–62.
- O Brien T, Ray E, Chatterton K, Khan MS, Chandra A (2013) Prospective randomized trial of hexylaminolevulinate photodynamic-assisted transurethral resection of bladder tumour (TURBT) plus single-shot intravesical mitomycin C vs conventional white-light TURBT plus mitomycin C in newly presenting non-muscle-invasi. BJU Int 112(8): 1096–1104.
- Gkritsios P, Hatzimouratidis K, Kazantzidis S (2014) Hexaminolevulinate-guided transurethral resection of non-muscle-invasive bladder cancer does not reduce the recurrence rates after a 2-year follow-up: A prospective randomized trial. Int Urol Nephrol 46(5): 927–933.
- Mariappan P, Rai B, El-Mokadem I, Anderson CH (2015) Real-life Experience: Early Recurrence with Hexvix Photodynamic Diagnosis-assisted Transurethral Resection of Bladder Tumour vs Good-quality White Light TURBT in New Non-muscle-invasive Bladder Cancer. Urology 86(2): 327–331.
- Gallagher KM, Gray K, Anderson CH, Lee H (2017) Real-life experience: recurrence rate at 3 years with Hexvix®photodynamic diagnosis-assisted TURBT compared with good quality white light TURBT in new NMIBC—a prospective controlled study. World J Urol 35(12): 1871-1877.
- Drăgoescu PO, Tudorache Ş, Drocaş AI (2017) Improved diagnosis and long-term recurrence rate reduction for non-muscle-invasive bladder cancer patients undergoing fluorescent hexylaminolevulinate photodynamic diagnosis. Rom J Morphol Embryol 58(4): 1279-1283.
- Lamm D, Persad R, Brausi M, Buckley R, Witjes JA (2014) Defining progression in nonmuscle invasive bladder cancer: It is time for a new, standard definition. J Urol 191(1): 20-27.
- Kamat AM, Cookson M, Witjes JA, Stenzl A, Grossman HB (2016) The impact of blue light cystoscopy with hexaminolevulinate (HAL) on progression of bladder cancer - A new analysis. Bl Cancer 2(2): 273-278.
- Klaassen Z, Li K, Kassouf W, Black PC, Dragomir A (2017) Contemporary cost-consequence analysis of blue light cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer. Can Urol Assoc J 11(6): 173–181.
- Garfield SS, Gavaghan MB, Armstrong SO, Jones JS (2013) The cost-effectiveness of blue light cystoscopy in bladder cancer detection: United States projections based on clinical data showing 4.5 years of follow up after a single hexaminolevulinate hydrochloride instillation. Can J Urol 20(2): 6682–6689.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...