Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Review Article(ISSN: 2641-1687) 
Medical Management of Castration Resistant Prostate Cancer (CRPC): Beyond Chemotherapy Volume 2 - Issue 5
Ganesh K Bakshi1, SK Raghunath2, Sudhir Rawal3, Ganesh Gopalakrishnan4, Sanjai K Addla5, Priya J6, Suyog C Mehta7, Mrinal Borgohain7 and Rajan Mittal7*
- 1Department of Urology, Tata Memorial Hospital, India
- 2Department of Urology, HCG Cancer Care, India
- 3Department of Urology, Rajiv Gandhi Cancer Institute and Research Centre, India
- 4Vedanayagam Hospital Pvt. Ltd., India
- 5Department of Urology, Apollo Hospital, India
- 6Department of Urology, Joshi Hospital, India
- 7Medical Affairs, Dr. Reddy’s Laboratories Ltd, India
Received: March 03, 2020; Published: March 12, 2020
Corresponding author: Dr. Rajan Mittal, MD, DM (Clinical Pharmacology) Director, Medical Affairs at Dr. Reddy’s Laboratories Ltd, 7-1-27, Ameerpet, Hyderabad, India
DOI: 10.32474/JUNS.2020.02.000146
Abstract
Prostate cancer is one of the leading causes of cancer-related mortality in men. Despite advances in treatment options, about 30-40% of patients develop the advanced disease in due course. Androgen deprivation is the standard first-line systemic therapy for men with advanced prostate cancer. Almost all patients with the metastatic disease go on to develop castration-resistant prostate cancer. The multiple therapeutic alternatives for castration-resistant prostate cancer, including abiraterone acetate, enzalutamide, cabazitaxel, immunotherapy with sipuleucel-T, radiopharmaceuticals and bone-targeted therapies (zoledronic acid, denosumab) along with docetaxel have made the decision-making process complex and challenging for clinicians. Even the strong pipeline of systemic therapies with a diverse array of mechanisms of action in prostate cancer have shown preliminary signs of clinical benefit, leading to more definitive phase III confirmatory trials. The review will relate the pathogenesis to the management of castrationresistant prostate cancer and look for the best therapy approaches in metastatic castration-resistant prostate cancer, needed to tackle the existing challenges effectively.
Keywords: Castration Resistant Prostate Cancer; Prostate Cancer; AR signaling inhibitor; metastatic; asymptomatic
Abbreviations: GLOBOCAN: Global Cancer Incidence, Mortality and Prevalence; CRPC: castration-resistant prostate cancer; mPC: metastatic prostate cancer; mCRPC: metastatic castration-resistant prostate cancer; NM-CRPC: non-metastatic CRPC; FDA: Food and Drug Administration; PSA: prostate-specific antigen; EAU: European Association of Urology; AA: abiraterone acetate; RECIST: Response Evaluation Criteria in Solid Tumors; ADT: androgen deprivation therapy; LBD: ligand binding domain; MAPK : mitogenactivated protein kinases; BRCA: breast cancer gene; ATM: ataxia telangiectasia mutated; AUA: American Urological Association; NEPC: Neuroendocrine prostate cancer
Introduction
Prostate cancer, with an estimated 1.4 million new cases and over 381,000 deaths annually, is a common cancer among men. It has the highest incidence among all cancers in 92 countries [1].
Prostate cancer
Incidence and mortality rates in South Central Asia were reported to be 5.0 and 3.3 per 100,000 person-years, respectively, as per Global Cancer Incidence, Mortality and Prevalence (GLOBOCAN) 2018 [2]. Approximately, 10 to 20% of newly diagnosed patients present with metastatic disease [3]. Almost all patients with metastatic prostate cancer (mPC) go on to develop castration-resistant prostate cancer (CRPC) [4]. A hospital-based survival study from Mumbai, India reported approximately 71% patients had mPC at diagnosis while another population based study reported that non-metastatic CRPC (NM-CRPC) represents only 3% of total PC population in Asian countries (China, India, Japan, Russia, and Turkey) [5,6]. Overall prognosis of metastatic castration-resistant prostate cancer (mCRPC) remains poor with a median survival of 1–2 years. Until recently, treatment options for mCRPC patients have been limited to docetaxel-based regimens [7,8]. Although docetaxel is associated with modest survival benefit, the availability of new frontline treatment regimens including sipuleucel-T, cabazitaxel, abiraterone acetate (AA) and enzalutamide, reporting longer survival, have extended the treatment options for men with mCRPC [9-11]. Recent phase III trials, SPARTAN, PROSPER and ARAMIS have demonstrated efficacy of the apalutamide, enzalutamide and darolutamide, respectively in NM-CRPC, a condition which has historically lacked level 1 evidence for treatment [12,13]. Thus, these Food and Drug Administration (FDA) approved new drugs can now be offered to the patients with NM-CRPC [14,15]. Selecting the correct treatment for mCRPC is complex as no head-to-head trials have been conducted and comparison between existing trials is difficult due to differences in study populations and lack of validated biomarkers. Multiple factors like treatment sequence, symptom burden, metastasis type, comorbidities and patient preference are taken into consideration for treatment decisions [16]. While new treatment paradigms are emerging and guidelines continue to evolve for management of patients with CRPC across the world, there is no standard of care in India. This review will relate the pathogenesis to management of CRPC and look for the best therapy approaches in mCRPC needed to tackle the existing challenges effectively.
Defining CRPC: guideline perspective
Several professional associations have developed guidelines for prostate cancer and have defined CRPC based on clinicopathologic, radiographic or biochemical progression (Table1) [17- 21]. The European Association of Urology (EAU) has standardized CRPC diagnosis based on four defining factors; castrate levels of serum testosterone (<1.7 nmol/L), 3 consecutive increases in prostate-specific antigen (PSA) one week apart resulting in two 50% increases above the nadir, a PSA >2 ng/mL and appearance of either two or more new bone lesions observed with bone scan or a soft tissue lesion using RECIST (Response Evaluation Criteria in Solid Tumors). It also recommends investigation of symptomatic progression alone though it is not sufficient to diagnose CRPC [18]. NM-CRPC refers to a rising PSA level under androgen deprivation therapy (ADT) with a castration level of testosterone and no clinically detectable metastatic disease while mCRPC is characterized by disease progression following surgical or medical castration in the presence of clinically detectable metastatic disease [18,22].
Table 1: CRPC Definition as per Guidelines.
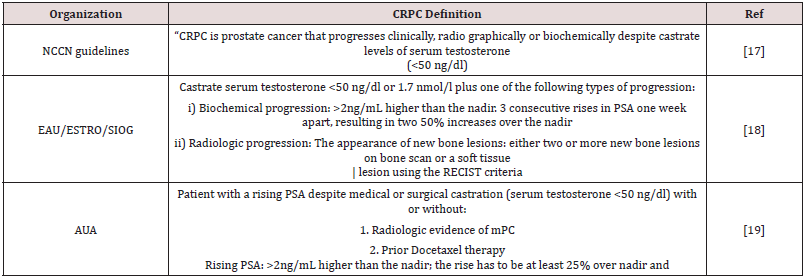
CRPC= Castration- resistant prostate cancer, RECIST= Response evaluation criteria in solid tumors, NCCN= National Comprehensive Cancer Network, PSA= Prostate-specific antigen, M0= Non metastatic, EAU/ESTRO/SIOG= European Association of Urology/ European Society for Radiotherapy and Oncology/International Society of Geriatric Oncology, AUA= American Urology Association, CUA= Canadian Urological Association, ESMO= European Society for Medical Oncology, mPC= metastatic prostate cancer.
Events in the course of prostate cancer progression to castration-resistant prostate cancer
The mechanisms and timing of development of CRPC are unclear and remain debatable. After the initial response to ADT, most of the patients with prostate cancer eventually develop the castration-resistant state. Approximately 33% of men with CRPC go on to develop metastases within 2 years of CRPC diagnosis. Various analyses indicate that progression to CRPC is multifactorial [23]. The “adaptation” model and the “clonal selection” model are two mechanisms that have been proposed to address this question. The adaptation model suggests that primary prostate cancer cells are homogeneous, in terms of their androgen requirement, and castration-resistant state emerges through genetic/epigenetic conversion of androgen-dependent cells to androgen-independent cells while the clonal selection model proposes that primary prostate cancer cells are heterogeneous with regards to their androgen requirement, of which a minority is a clone of preexisting castration-resistant cells [23]. The progression of tumor growth and development of metastases despite reduction of serum androgens to ‘castrate’ levels is dependent upon the utilization of adaptive cell-survival pathways. Mechanisms contributing to CRPC and its progression include various androgen/androgen receptor dependent and independent pathways (Figure1).
Figure 1: Mechanisms contributing to CRPC progression.
ACTH- Adrenocorticotropic hormone, PARP- polyADP-ribose polymerases, PI3K - phosphatidylinositol 3-kinase, Akt
-Protein Kinase B, Bcl-2 -Antiapoptotic protein, Bad - BH3 proteins Sensitizer , mTOR-mammalian target of rapamycin,
MAPK/ERK- mitogen- activated protein kinases.
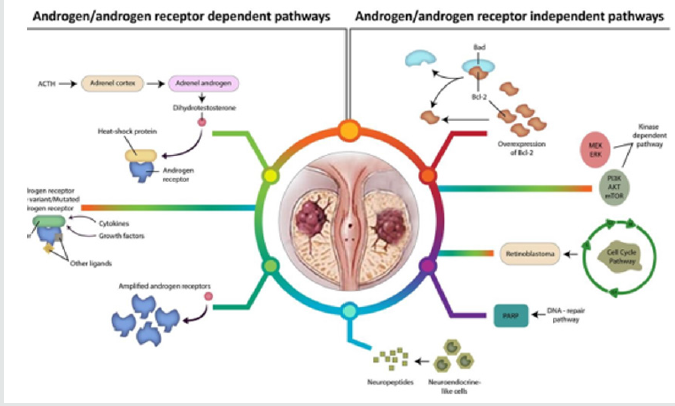
Androgen/androgen receptor dependent pathway
Intraprostatic steroidogenesis: Studies have suggested that testosterone levels in prostate of men with CRPC are equivalent of those found in non-castrate patients. Up regulation of enzymes involved in androgen synthesis in prostate cancer cells is hypothesized to be the underlying mechanism of increased testosterone in prostate of CRPC patients [24].
Increased sensitivity of androgen receptor (AR): In CRPC patients, activation of the AR signaling pathways occurs through the genetic phenomena that result in increased sensitivity of AR to very low levels of androgens [24,25].
AR mutations: Mutations of the AR gene have been found in 10% to 30% of CRPC patients. These mutations lead to changes in ARs that affect the ligand binding, reduce specificity and increase promiscuity of binding to non-androgen ligands [24].
AR splice variants: The majority of AR splice variants (AR-Vs) identified to date in prostate cancer cells are generated through insertion of downstream cryptic exon or deletions within the ligand binding domain (LBD), producing truncated AR lacking LBD, rendering them impervious to commonly utilized anti-androgen agents [26,27].
Androgen/androgen receptor independent pathway
Altered apoptotic pathway: In particular, B-cell lymphomaextra- large (Bcl-xL) expression increases during prostate cancer progression from primary to castration-resistant state. Activated ligand-independent AR signaling has been found to be associated with increased levels of Bcl-xL [28].
Kinase-dependent pathway: Deregulation of the kinasedependent signaling pathways, MAPK/ERK (mitogen-activated protein kinases) or PI3K-AKT-mTOR (phosphoinositide-3-kinase- AKT-mammalian target of rapamycin), has been found to be associated with mCRPC in >50% of patients. These pathways are involved in a variety of biological processes such as cell survival, proliferation, differentiation, apoptosis, survival, invasion, and migration as well as angiogenesis [29].
Alteration in DNA repair pathway: Deleterious germ line or somatic aberrations in DNA damage repair genes have been found in 19% and 23% of primary prostate cancer and mCRPC respectively, with breast cancer gene (BRCA), cyclin-dependent kinase 12 (CDK12) & ataxia telangiectasia mutated (ATM) as the commonly altered gene [29].
Alteration in cell cycle pathway: Retinoblastoma (RB) gene product is a critical inhibitor of transition from G1–S phase, preventing premature cell division [30]. RB gene loss is reported in 21% of CRPCs and its inactivation promotes the reprogramming of differentiated cells to a pluripotent state [29].
Treatment-related neuroendocrine prostate cancer: Neuroendocrine prostate cancer (NEPC) is a subtype of prostate cancer that rarely arises de novo, but commonly arises after hormonal therapy [31]. It also has aggressive biologic behavior and poor outcomes
Management of CRPC
The treatment of prostate cancer has been rapidly changing and is still evolving. Despite a high initial response rate (80-90%) with ADT, nearly all patients with prostate cancer eventually develop progressive disease. Persistent AR signaling is an important driving force for their progression and thus, AR signaling axis represents the most important therapeutic target for the treatment of both castration naïve prostate cancer and CRPC. In castration-naïve prostate cancer, patients with biochemical recurrence should be risk stratified based on the Gleason score, time to biochemical recurrence and PSA doubling time. Evidence suggests that only high-risk biochemical recurrence patients should be considered for ADT. In addition, current practice supports the use of ADT in the setting of metastatic hormone naïve prostate cancer [32].
Therapy of men with non-metastatic castration-resistant
prostate cancer: After almost 20 years of negative outcomes
in clinical trials involving men with NM-CRPC, AR antagonists
namely apalutamide, enzalutamide and darolutamide have
shown favorable outcomes in three different trials (SPARTAN,
PROSPER, ARAMIS trials) [3,12]. Based on results of these trials,
US FDA has approved these drugs (apalutamide, enzalutamide and
darolutamide) for the treatment of NM-CRPC. In the SPARTAN trial,
1,207 men with NM-CRPC at high risk of disease progression were
randomized in a 2:1 fashion to receive either apalutamide 240 mg
orally daily with ADT (n =806) or placebo with ADT (n=401) [12].
The median metastasis-free survival (MFS) for patients treated
with apalutamide was 40.5 months versus 16.2 months for the
placebo arm (HR 0.28, 95% CI 0.23–0.35; P<0.001). Apalutamide
also reduced the risk of symptomatic progression by 55% (HR 0.45,
95% CI: 0.32–0.63; P<0.001). Estimated rates of any adverse events
(AEs) that led to discontinuation of the trial regimen were 10.6%
for apalutamide and 7.0% for placebo, respectively. Apalutamidetreated
patients had a higher incidence of rash (23.8% vs. 5.5%),
fracture (11.7% vs. 6.5%) and hypothyroidism (8.1% vs. 2.0%)
than with placebo. In another randomized phase III PROSPER
trial, a total of 1,401 men with NM-CRPC were randomized in a
2:1 fashion to receive enzalutamide 160 mg or placebo once daily
[13]. Enzalutamide significantly increased MFS (36.6 months
vs. 14.7 months; HR 0.29; P < .0001), time to PSA progression
(37.2 months vs. 3.9 months; HR 0.07; P < .0001) and time to first
use of new antineoplastic therapy (39.6 months vs. 17.7 months;
HR 0.21; P < .0001) compared to placebo [13]. The estimated rates
of any AEs leading to discontinuation were 10% for enzalutamide and 8% for placebo. The overall incidence of AEs was higher with
enzalutamide than with placebo (any grade: 87% vs. 77%; grade
⩾3: 31% vs. 23%; serious: 24% vs. 18%) [13].
In 2019, the results of double-blind, placebo-controlled,
phase III ARAMIS trial evaluating darolutamide for the treatment
of advanced prostate cancer were published [33]. A total of 1509
patients with NM-CRPC were randomized in a ratio of 2:1 to receive
darolutamide 600 mg (two 300 mg tablets) twice-daily or placebo,
while continuing androgen deprivation therapy. Darolutamide
significantly increased MFS (40.4 months vs. 18.4 months; HR
0.41; 95% CI 0.34–0.50; P< .0001), overall survival (HR 0.71, 95%
CI 0.50–0.99; 2-sided p=0.045), and time to pain progression (HR
0.65; 95% CI 0.53–0.79; 2-sided p<0.0001) compared to placebo.
The estimated rates of any AEs leading to discontinuation were
8.9% for darolutamide and 8.7% for placebo. The incidence
rate of treatment-emergent AEs were also comparable between
darolutamide and placebo arms (≥5% frequency for grade ⩾3
AEs). American Urological Association (AUA) guidelines 2018
on prostate cancer have recommended use of apalutamide or
enzalutamide in patients with NM-CRPC at high risk for developing
metastatic disease, along with continued androgen deprivation.
Those patients who do not want or cannot have one of the standard
therapies should either be under observation with continued
androgen deprivation or may be offered treatment with a secondgeneration
androgen synthesis inhibitor (i.e. AA + prednisone), if
at high risk for developing metastatic disease. Although similar in
mechanism, darolutamide does not cross the blood-brain barrier
and thus confers a much lower seizure risk than enzalutamide or
apalutamide [19].
Therapy of men with metastatic castration-resistant
prostate cancer: Currently, the guidelines from AUA and
National Comprehensive Cancer Network (NCCN) recommend
FDA-approved therapies in mCRPC patients to improve survival
and palliate symptoms. Treatment options include sipuleucel-T,
docetaxel-based chemotherapy and novel hormonal therapies
[such as enzalutamide and AA plus prednisone (AAP) [17,19,34].
If patients fail on these therapies, alternative treatment options
include radiopharmaceutical therapy (radium-223 dichloride) and
cabazitaxel [34]. Both taxane-based chemotherapy and AA require
concomitant use of corticosteroids [8,35]. Guidelines from the AUA,
EAU and NCCN recommend prednisolone while current clinical
trials suggest dexamethasone may be more potent compared to
prednisolone in the treatment of mCRPC [17-19,36-38]. The NCCN
2019 recommends sipuleucel-T for asymptomatic patients with
mCRPC having Eastern Cooperative Oncology Group performance
status of 0–1 and have life expectancy of more than six months [17].
In addition, pembrolizumab may also be offered to patients who
have progressed following at least one systemic therapy for M1
CRPC (presence of metastases) [17].
Newer anti-androgens, like enzalutamide and AA, were
developed to overcome the efficacy limitation of first-generation
anti-androgens in the setting of AR over-expression or of specific
mutations in the AR ligand-binding domain. Enzalutamide has fiveto-
eight times higher binding affinity for AR than bicalutamide; it inhibits AR nuclear translocation and impairs binding of AR to
androgen response elements on DNA and their activation [39].
Enzalutamide is also active against prostate cancer cell lines
bearing the W741C AR point mutation that is known to confer
resistance to bicalutamide [40]. It has shown encouraging activity
both among chemotherapy-experienced and chemotherapy-naive
mCRPC patients [41], although a clinical study demonstrated that
proportion of patients achieving PSA decline in excess of 50% with
enzalutamide is higher among the chemotherapy naïve patients
(57%; 95% CI 44-69%) than in chemotherapy experienced patients
(36%; 95% CI 25-48%) at 12 weeks [42].
A network meta-analysis of eight randomized controlled trials
was conducted by Kang et al. to compare the efficacy of novel
AR-targeted agents in patients with mCRPC (5 studies in the prechemotherapy
and 3 in the post-chemotherapy settings) [43].
This meta-analysis found significantly improved overall survival
(OS) with enzalutamide and AA as compared to control arms.
Network meta-analysis also showed that enzalutamide ranked
first as the most efficacious agent in improving OS (HR = 0.71)
and AA was ranked second in this regard (HR=0.78). Further,
enzalutamide significantly improved progression-free survival
(PFS) in comparison with control groups (HR = 0.36), and a trend
towards improved PFS was observed with AA. In comparison to
control groups, Enzalutamide (HR = 0.20) and AA (HR=0.56) were
significantly associated with prolonged times to PSA progression.
Treatment of chemotherapy exposed mCRPC patients:
In 2017, Rocha et al. conducted a retrospective cohort study to
investigate the impact of AA with and without prior docetaxel
chemotherapy on the survival of patients with mCRPC using
Quebec public health care administrative databases [44]. The
median age at initiation of AA therapy was 75 years for the postdocetaxel-
chemotherapy group and 80 years for the other patient
group, without chemotherapy. The median survival in the two
groups was 12 and 14 months (log-rank test p = 0.8), respectively.
Risk of death was similar in the 2 groups (adjusted HR 0.89 [95%
CI 0.57-1.38]). This study showed that effectiveness of AA in older
patients who were ineligible for chemotherapy was similar to that
of patients with prior docetaxel exposure. Overall, the real-world
survival benefits of AA were similar to those in the COU-AA-301
trial. Similarly, in AFFIRM study, median OS was 18.4 months with
enzalutamide (n = 800) versus 13.6 months in the placebo arm (n
= 399) in patients of mCRPC who had relapsed following docetaxelbased
chemotherapy (HR for death in the enzalutamide arm, 0.63;
95% CI, 0.53 to 0.75; P<0.001) [10]. In each study group, about 92%
of men had bone metastases at baseline. Compared with placebo,
enzalutamide reduced the risk of death by 33% (HR 0.67; 95% CI,
0.52–0.87) in those with >20 and 41% (HR 0.59; 95% CI, 0.46–0.75)
in those with ≤20 bone lesions, respectively. Enzalutamide was also
superior to placebo for time to first reportable skeletal-related
event (SRE) (16.7 vs. 13.3 months, respectively; HR 0.69; 95% CI,
0.57– 0.84; P < 0.001). Data from STRIVE trial has demonstrated
the superiority of enzalutamide over bicalutamide in patients with
or without metastatic CRPC (n=396) who progressed despite ADT.
Enzalutamide reduced the risk of progression or death by 76% versus bicalutamide (HR 0.24; 95% CI, 0.18 to 0.32; P < .001) [45].
Median time to PSA progression (HR 0.19; P < 0.001) and PFS
(median PFS duration: 16.5 vs. 5.5 months; HR 0.24, P < 0.001)
were significantly improved with enzalutamide as compared to
bicalutamide. The side effects were comparable between groups;
grade ≥3 AEs and treatment-related deaths occurred in 36% of
patients and in 3% of patients in each group, respectively.
Radium-223 (Ra-223) is an alpha particle emitter and calciummimetic
that is used for therapy of patients with CRPC having
symptomatic bone metastasis. Ra-223 delivers high energy but
short-range radiation, limiting damage to normal tissues [46]. Ra-
223 improves OS and delays time to first symptomatic skeletalrelated
events in mCRPC patients [47]. In phase I/IIa clinical trial,
Ra-223 plus docetaxel combination, in comparison to docetaxel
alone, prolonged time to progression of PSA (median time to
progression, 6.6 vs. 4.8 months), improved PFS (median PFS 12.0
months vs. 9.3 months) and prolonged time to progression of total
alkaline phosphatase (ALP) and bone ALP (9.0 vs. 6.9 and 9.3 vs. 7.4
months, respectively) [48].
Treatment of chemotherapy naive mCRPC patients: Enzalutamide and AA with prednisone have also received the highest recommendation (category1) in the NCCN guidelines for the first line therapy of patients with asymptomatic, chemotherapy naive mCRPC [17]. Cluster ranking of one of the meta-analysis involving 18 studies in pre-chemotherapy settings demonstrated that enzalutamide and AA were ideal agents for improving PFS and OS in chemotherapy-naïve mCRPC patients with a low risk of causing severe AEs [49]. In double-blind, phase 3 PREVAIL trial, the risk of radiographic progression (81% risk reduction; HR 0.19; 95% CI, 0.15 to 0.23; P<0.001) and death (29% reduction in the risk of death; HR 0.71; 95% CI, 0.60 to 0.84; P<0.001) were significantly reduced by enzalutamide vs. placebo in patients with chemotherapy naive mCRPC. Enzalutamide also delayed the initiation of chemotherapy in these patients [50]. The results from long-term overall survival and safety analyses of the phase 3 PREVAIL study showed significant OS benefit with 32.4 months in the enzalutamide arm vs. 30.2 months in the placebo arm at the median follow up time of 69 months. In overall survival analysis, enzalutamide resulted in 17% reduction in the risk of death (HR 0.83, 95% CI 0.75-0.93, p=0.008) [51]. Similarly, in the COU-AA-302 phase 3 trial, overall survival for men with chemotherapy-naive mCRPC was significantly longer in the AA plus prednisone (n = 542) than in the prednisone alone (n = 546) group (34·7 months [95% CI 32·7–36·8] vs. 30·3 months [95% CI 28·7–33·3]; HR 0·81 [95% CI 0·70–0·93]; p=0·0033) [52].
Treatment of asymptomatic or minimally symptomatic mCRPC patients: The autologous, active cellular immunotherapy, sipuleucel-T which utilizes patient’s own antigen-presenting cells, is an FDA approved agent for therapy of asymptomatic or minimally symptomatic mCRPC [17]. Randomized Phase III trial (D9901) evaluating the effect of sipuleucel-T in men with asymptomatic mCRPC has shown improvement in mean survival from 21.4 months to 25.9 months. Study result revealed that the safety profile of sipuleucel-T was similar to that of placebo [53]. In D9902A trial, sipuleucel-T showed non-significant 21% reduction in risk of death compared to placebo among men with asymptomatic mCRPC (HR 1.27; 95%CI, 0.78‐2.07; P = .33). Additionally, when data from the D9901 and D9902A trials were integrated, significant increase in median OS for the sipuleucel-T group versus placebo was observed with a benefit of 4.3 months (HR 1.50; 95% CI 1.10–2.05; log rank p = 0.01) [54]. In 2016, a randomized, double-blind, phase 2 TERRAIN trial compared enzalutamide to bicalutamide in patients with asymptomatic/mildly symptomatic mCRPC who progressed despite ADT. Enzalutamide significantly increased the median progression-free survival (15·7 months [95% CI, 11·5-19·4]) versus bicalutamide (5·8 months [95% CI, 4·8-8·1]; hazard ratio 0·44 [95% CI 0·34-0·57]; p<0·0001). Median follow-up time was also longer for the patients treated with enzalutamide group (20 months) compared with 16.7 months in the bicalutamide group [55].
Selecting appropriate therapies in the mCRPC patient
As CRPC progresses to advanced stage (i.e. mCRPC), a multidisciplinary approach is needed to improve survival and quality of life of the patients. At the same time, little is known about optimal sequencing and combination strategies, and how cross resistance can evolve for subsequent treatments [56]. Front line therapies include docetaxel, sipuleucel-T, AA/prednisone, enzalutamide and Ra-223. The AUA guidelines consider different scenarios based on chemotherapy naïve or exposed mCRPC patients [19].
Chemo-naïve patients having:
a) Asymptomatic or minimally symptomatic mCRPCmay
receive AAP, enzalutamide, or docetaxel chemotherapy and
sipuleucel-T immunotherapy
b) Symptomatic mCRPC and good performance status- may
receive AAP, enzalutamide, or docetaxel
c) Symptomatic mCRPC, poor performance status- may
receive AAP or enzalutamide.
Chemo-exposed with docetaxel:
a) Symptomatic mCRPC, good performance status- may
receive AAP, cabazitaxel, or enzalutamide
b) Symptomatic mCRPC, poor performance status- should
not be offered AAP
However, grade A evidence is lacking for combinations or sequential use of the above-mentioned therapies, apart from the use of Ra-223 after docetaxel, leaving clinicians with imperfect guidance on treatment selection for individual patients [57]. Despite limitations, some consistent observations have arisen from studies. First, cross-resistance occurs between the new androgen-receptor– targeting agents. The rate of response to AA therapy after treatment with enzalutamide is less than 10%, whereas the response rate for enzalutamide after AA is 15 to 30% [58-60]. However, major progress can be expected by the validation of predictive biomarkers of cross-resistance between these treatments and might lead to more rational sequencing strategies with more accurate patient selection. Although cross-resistance between the two agents have been observed in a majority of patients, but some still get benefit from enzalutamide treatment following ≥24 week of AA plus prednisone treatment [61]. The benefit from taxanes appears to be diminished after treatment with AA or enzalutamide, as compared with the benefit in patients who have not received such treatment, although taxanes remain active [62]. Guidance in treatment decisions for such patients is limited as large, prospective, randomized trials with taxanes in men having already treated with AA or enzalutamide, are not available. Potential synergistic activity between agents has led to the evaluation of multiple combinations. No combination has been proven to have superior efficacy in mCRPC to date; nine randomized phase 3 trials enrolling more than 10 000 patients failed to prove the benefit of any docetaxel combination. The phase III ALpharadin in SYMPtomatic Prostate CAncer (ALSYMPCA) trial has reported that Ra-223 should be considered as a treatment option for patients with CRPC and symptomatic bone metastases. Owing to low toxicity rates of Ra- 223 observed in ALSYMPCA and non-overlapping mechanism of action, its combinations with AA or enzalutamide, have attracted interest. In an expanded access program (EAP) from USA, no new safety concerns were reported with Ra-223; safety was maintained with AA or enzalutamide [63, 64]. The ongoing PEACE-3 trial is now evaluating the combination of enzalutamide and Ra-223 in mildly symptomatic mCRPC (EUDRACT 2014-001787-36). However, it is not a foregone conclusion that any of these combinations will improve outcome. In a neoadjuvant study of 24 weeks, the combination of enzalutamide, AA, and leuprolide acetate showed pathologic down staging in only 30% of patients relative to 52% of patients receiving AA and leuprolide acetate, calling into question the synergy between these novel hormonal agents [65].
The Post-Abiraterone /Post-Enzalutamide Space
The question of what to do with patients with mCRPC who had
failed treatment with AA is currently subject to debate. Since 2004,
docetaxel has been viewed as standard of care for chemotherapy
naïve mCRPC patients, but results are mixed at best. The de Bono
group has reported diminished response to docetaxel in patients
who have already received AA treatment [66]. There are no large
trials in this setting so conclusions must be tempered until more
data are available. Fizazi and colleagues have reported relatively
high PSA response rates for cabazitaxel/prednisone in patients
previously treated with AA [67]. However, there is need to learn
about characteristics of the treated patients and response durability
as this study was only published in abstract form.
In 2014, a study by Schrader et al. [59] indicated that the
response to enzalutamide post-AA/post-docetaxel was only
modest compared to the patients treated post-docetaxel alone [60].
However, one study by Schler et al. have reported positive results
for enzalutamide in patients previously treated with docetaxel. In
this study, 56% of men achieved a >50% decline in PSA and only
17% had no PSA response [68]. Thomson and colleagues have also
reported enzalutamide activity following failure of docetaxel and
AA in mCRPC. This activity was more pronounced in those who
have responded to AA [69].
Newer Treatments in Managing CRPC
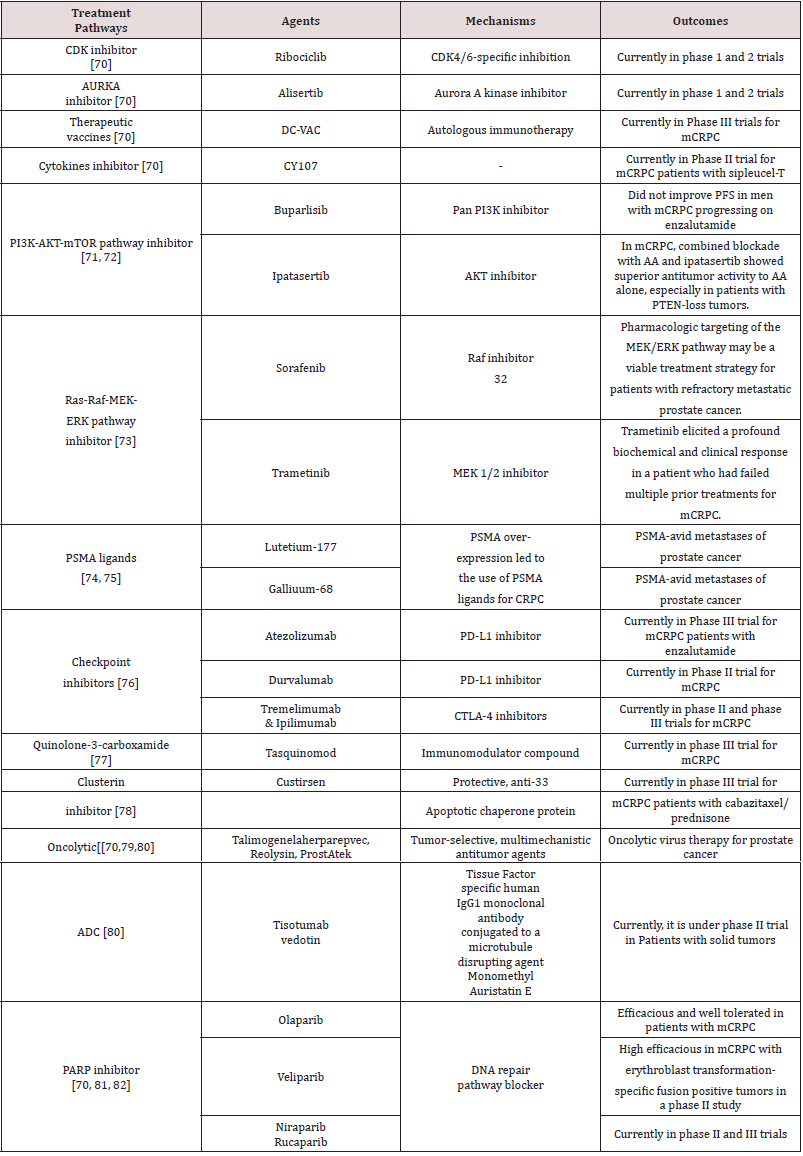
The current treatment strategies are still less than satisfactory and have paved way for the novel agents in effectively treating CRPC. A considerable number of novel agents against mCRPC based on diverse mechanisms are currently under investigation worldwide (Table2). A number of novel agents (buparlisib, ipataserib, olaparib, palbocicilib etc) that act against mCRPC based on diverse mechanism (PI3K-AKT-mTOR pathway inhibitor, PARP inhibitor and CDK inhibitor) are currently under investigation worldwide. List of newer treatments along with their mechanism of action are shown in the Table 2 [70-82].
Conclusion
The management of advanced prostate cancer has undergone a revolution over the last decade with the emergence of new science and evidences in novel bone-targeted agents, immunotherapy, chemotherapy and AR pathway-targeted agents. In clinical trials, AA, enzalutamide, and Ra-223 improved the radiographic progressionfree survival and overall survival in patients with mCRPC compared with placebo. Overall, enzalutamide was found to be most effective treatment option for mCRPC patients in both chemotherapy naïve and pre-treated setting. In addition to enzalutamide, Grade A evidence also supports the use of sipuleucel-T, AA–prednisone, docetaxel, and Ra-223 in chemotherapy naïve setting while in pre-treated patients, use of AA–prednisone, cabazitaxel, and Ra- 223 has been supported. However, Grade A evidence is lacking for combination or for sequential use of these therapies, apart from the use of Ra-223 after docetaxel. Prospective randomized clinical trials addressing the best therapy approaches in mCRPC are required to determine evidence-based sequencing strategies.
Acknowledgement
Authors thank Work Sure India for medical writing and editing support
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- Fitzmaurice C, Akinyemiju TF, AlLami FH, Alam T, Alizadeh-Navaei R, et al. (2018) Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 4(11): 1553-1568.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6): 394-424.
- Leonel Almeida P, Jorge Pereira B (2018) Local Treatment of Metastatic Prostate Cancer: What is the Evidence So Far ? Prostate Cancer 2018.
- Saad F, Fizazi K (2015) Androgen Deprivation Therapy and Secondary Hormone Therapy in the Management of Hormone-sensitive and Castration-resistant Prostate Cancer. Urology 86(5): 852-861.
- Balasubramaniam G, Talole S, Mahantshetty U, Saoba S, Shrivastava S (2013) Prostate cancer: a hospital-based survival study from Mumbai, India. Asian Pac J Cancer Prev 14(4): 2595-2598.
- Liede A, Arellano J, Hechmati G, Bennett B, Wong S (2013) International prevalence of nonmetastatic (M0) castration-resistant prostate cancer (CRPC). J Clin Oncology 31(15_suppl).
- Autio KA, Dreicer R, Anderson J, Garcia JA, Alva A, et al. (2018) Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA oncol 4(10): 1344-1351.
- Baciarello G, Gizzi M, Fizazi K (2018) Advancing therapies in metastatic castration-resistant prostate cancer. Expert Opin Pharmacother 19(16): 1797-1804.
- Handy CE, Antonarakis ES (2018) Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol 14(10): 907-917.
- Siemens DR, Klotz L, Heidenreich A, Chowdhury S, Villers A, et al. (2018) Efficacy and safety of enzalutamide vs bicalutamide in younger and older patients with metastatic castration resistant prostate cancer in the TERRAIN trial. J Urol 199(1): 147-154.
- Oudard S, Fizazi K, Sengeløv L, Daugaard G, Saad F, et al. (2017) Cabazitaxel versus docetaxel as first- line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial—FIRSTANA. J Clin Oncol 35(28): 3189-3197.
- Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, et al. (2018) Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 378(15): 1408-1418.
- Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, et al. (2018) Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 378(26): 2465-2474.
- Alpajaro SIR, Harris JAK, Evans CP (2019) Non-metastatic castration resistant prostate cancer: a review of current and emerging medical therapies. Prostate Cancer Prostatic Dis 22(1): 16-23.
- Rhea LP, Gupta B, Aragon-Ching JB (2019) Enzalutamide: a new indication for nonmetastatic castration-resistant prostate cancer. Asian J Androl 21(2): 107-108.
- Crawford ED, Higano CS, Shore ND, Hussain M, Petrylak DP (2015) Treating Patients with Metastatic Castration Resistant Prostate Cancer: A Comprehensive Review of Available Therapies. J Urol 194(6): 1537-1547.
- Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, et al. (2019) Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 17(5): 479-505.
- Mottet N, Van Den Bergh RCN, Briers E, Bourke L, Cornford P, et al. (2019) EAU Guidelines.
- Lowrance WT, Murad MH, Oh WK, Jarrard DF, Resnick MJ, et al. (2018) Castration- Resistant Prostate Cancer: AUA Guideline Amendment 2018. J Urol 200(6): 1264-1272.
- Saad F, Aprikian A, Finelli A, Fleshner NE, Gleave M, et al. (2019) 2019 Canadian Urological Association (CUA)-Canadian Uro Oncology Group (CUOG) guidelines for the management of castration-resistant prostate cancer (CRPC). Can Urol Assoc J 13(10): 307-314.
- Horwich A, Parker C, de Reijke T, Kataja V, ESMO Guidelines Working Group (2013) Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 129(33).
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. (2014) EAU guidelines on prostate cancer part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65(1): 124-137.
- Ahmed M, Li LC (2013) Adaptation and clonal selection models of castration-resistant prostate cancer: current perspective. Int J Urol 20(4): 362-371.
- Amaral TM, Macedo D, Fernandes I, Costa L (2012) Castration-resistant prostate cancer: mechanisms, targets, and treatment. Prostate Cancer 2012.
- Armstrong CM, Gao AC (2016) Adaptive pathways and emerging strategies overcoming treatment resistance in castration resistant prostate cancer. Asian J Urol 3(4): 185-194.
- Egan A, Dong Y, Zhang H, Qi Y, Balk SP, (2014) Castration-resistant prostate cancer: adaptive responses in the androgen axis. Cancer Treat Rev 40(3): 426-433.
- Wadosky KM, Koochekpour S (2017) Androgen receptor splice variants and prostate cancer: From bench to bedside. Oncotarget 8(11): 18550-18576.
- Song IS, Jeong YJ, Kim J, Seo KH, Baek NI, et al. (2020) Pharmacological inhibition of androgen receptor expression induces cell death in prostate cancer cells. Cell Mol Life Sci 1: 1-1.
- Cattrini C, Zanardi E, Vallome G, Cavo A, Cerbone L, et al. (2017) Targeting androgen-independent pathways: new chances for patients with prostate cancer? Crit Rev Oncol Hematol 118: 42-53.
- Weinberg RA (2013) The Biology of Cancer: Second International Student Edition: WW Norton & Company pp. 281-303.
- Chen R, Dong X, Gleave M (2018) Molecular model for neuroendocrine prostate cancer progression. BJU int 122(4): 560-570.
- Moul JW (2015) Hormone naive prostate cancer: predicting and maximizing response Asian J Androl 17(6): 929-935.
- Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, et al. (2019) Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 380(13): 1235-1246.
- Penson DF, Lin DW, Karsh L, Quinn DI, Shevrin DH, et al. (2016) Treatment registry for outcomes in patients with castration-resistant prostate cancer (TRUMPET): a methodology for real-world evidence and research. Future Oncol 12(23): 2689-2699.
- Nevedomskaya E, Baumgart SJ, Haendler B (2018) Recent advances in prostate cancer treatment and drug discovery. Int J Mol Sci 19(5): 1359.
- De Santis M, Saad F (2016) Practical Guidance on the Role of Corticosteroids in the Treatment of Metastatic Castration-resistant Prostate Cancer. Urology 96: 156-164.
- Venkitaraman R, Lorente D, Murthy V, Thomas K, Parker L, et al. (2015) A randomised phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol 67(4): 673-679.
- Schalken J, Fitzpatrick JM (2016) Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int 117(2): 215-225.
- Imamura Y, Sadar MD (2016) Androgen receptor targeted therapies in castration-resistant prostate cancer: Bench to clinic. Int J Urol 23(8): 654-665.
- Menon MP, Higano CS (2013) Enzalutamide, a second-generation androgen receptor antagonist: development and clinical applications in prostate cancer. Curr Oncol Rep 15(2): 69-75.
- Bennett LL, Ingason A (2014) Enzalutamide (Xtandi) for patients with metastatic, resistant prostate cancer. Ann Pharmacothe 48(4): 530-537.
- Kang M, Jeong CW, Kwak C, Ku JH, Kim HH (2017) Comparing the clinical efficacy of abiraterone acetate, enzalutamide, and orteronel in patients with metastatic castration-resistant prostate cancer by performing a network meta-analysis of eight randomized controlled trials. Oncotarget 8(35): 59690-59697.
- Rocha J, Aprikian AG, Vanhuyse M, Cury FL, Hu J, et al. (2017) Impact of abiraterone acetate with and without prior docetaxel chemotherapy on the survival of patients with metastatic castration-resistant prostate cancer: a population-based study. CMAJ open 5(1): 265-272.
- Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, et al. (2016) Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. J Clin Oncol 34(18): 2098-2106.
- Chopra S, Rashid P (2015) Management of castration-resistant (advanced) prostate cancer (CRPC): rationale, progress and future directions. Aust Fam Physician 44(5): 302-305.
- Parker C, Heidenreich A, Nilsson S, Shore N (2018. Prostate Cancer Prostatic Dis 21(1): 37-47.
- Morris MJ, Loriot Y, Sweeney CJ, Fizazi K, Ryan CJ, et al. (2019) Radium-223 in combination with docetaxel in patients with castration-resistant prostate cancer and bone metastases: a phase 1 dose escalation/randomised phase 2a trial. Eur J Cancer 114: 107-116.
- Wang Y, Zhang H, Shen W, He P, Zhou Z (2018) Effectiveness and tolerability of targeted drugs for the treatment of metastatic castration-resistant prostate cancer: a network meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol 144(9): 1751-1768.
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, et (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371(5): 424-433.
- Armstrong A, Tombal B, Saad F, Parli T, Phung D, et al. (2019) Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC): Long-term overall survival and safety analyses of the phase 3 PREVAIL study. Eur Urol Suppl 18(1): 1217-1218.
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, et al. (2015) Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16(2): 152-160.
- Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, et al. (2006) Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 24(19): 3089-3094.
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, et al. (2009) Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115(16): 3670-3679.
- Shore ND, Chowdhury S, Villers A, Klotz L, Siemens DR, et al. (2016) Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol 17(2): 153-163.
- Fitzpatrick JM, Bellmunt J, Fizazi K, Heidenreich A, Sternberg CN, et al. (2014) Optimal management of metastatic castration-resistant prostate cancer: highlights from a European Expert Consensus Panel. Eur J Cancer 50(9): 1617-1627.
- Sartor O, de Bono JS (2018) Metastatic Prostate Cancer. N Engl J Med 378: 645-657.
- Smith MR, Saad F, Rathkopf DE, Mulders PFA, de Bono JS, et al. (2017) Clinical Outcomes from Androgen Signaling-directed Therapy after Treatment with Abiraterone Acetate and Prednisone in Patients with Metastatic Castration-resistant Prostate Cancer: Post Hoc Analysis of COU-AA-302. Eur Urol 72(1): 10-13.
- Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, et al. (2013) Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol 24(7): 1802-1807.
- Schrader AJ, Boegemann M, Ohlmann CH, Schnoeller TJ, Krabbe LM, et al. (2014) Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol 65(1): 30-36.
- De Bono JS, Chowdhury S, Feyerabend S, Elliott T, Grande E, et al. (2018) Antitumour Activity and Safety of Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer Previously Treated with Abiraterone Acetate Plus Prednisone for >/=24 weeks in Europe. Eur Urol 74(1): 37-45.
- De Bono JS, Smith MR, Saad F, Rathkopf DE, Mulders PFA, et al. (2017) Subsequent Chemotherapy and Treatment Patterns After Abiraterone Acetate in Patients with Metastatic Castration-resistant Prostate Cancer: Post Hoc Analysis of COU-AA-302. Eur Urol 71(4): 656-664.
- Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, et al. (2014) Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 15(7): 738-746.
- Sartor O, Vogelzang NJ, Sweeney C, Fernandez DC, Almeida F, et al. (2018) Radium-223 Safety, Efficacy, and Concurrent Use with Abiraterone or Enzalutamide: First U.S. Experience from an Expanded Access Program. Oncologist 23(2): 193-202.
- Efstathiou E, Davis JW, Titus MA, Chapin BF, Zurita AJ, et al. (2016) Neoadjuvant enzalutamide (ENZA) and abiraterone acetate (AA) plus leuprolide acetate (LHRHa) versusAA+ LHRHa in localized high-risk prostate cancer (LHRPC). J Clin Oncol 34 (15_suppl): 5002.
- Mezynski J, Pezaro C, Bianchini D, Zivi A, Sandhu S, et al. (2012) Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol 23(11): 2943-2947.
- Silberstein JL, Pal SK, Lewis B, Sartor O (2013) Current clinical challenges in prostate Transl Androl Urol 2(3): 122-136.
- Scher HI, Beer TM, Higano CS, Anand A, Taplin M-E, et al. (2010) Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375(9724): 1437-1446.
- Thomson D, Charnley N, Parikh O (2014) Enzalutamide after failure of docetaxel and abiraterone in metastatic castrate resistant prostate cancer (mCRPC): Results from an expanded access program. J Clin Oncol 32(4_suppl): 188.
- Yoo S, Choi SY, You D, Kim CS (2016) New drugs in prostate cancer. Prostate Int 4(2): 37- 42.
- Armstrong AJ, Halabi S, Healy P, Alumkal JJ, Winters C, et al. (2017) Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur J Cancer 81: 228-236.
- De Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, et al. (2019) Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin Cancer Res 25(3): 928-936.
- Nickols NG, Nazarian R, Zhao SG, Tan V, Uzunangelov V, et al. (2019) MEK-ERK signaling is a therapeutic target in metastatic castration resistant prostate cancer.Prostate Cancer Prostatic Dis 22(4): 531-538.
- Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, et al. (2013) Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res 19(18): 5182-5191.
- Taghizadeh H, Marhold M, Tomasich E, Udovica S, Merchant A, et al. (2019) Immune checkpoint inhibitors in mCRPC-rationales, challenges and perspectives. OncoImmunology 8(11).
- Virgolini I, Decristoforo C, Haug A, Fanti S, Uprimny C (2018) Current status of theranostics in prostate cancer. Eur J Nucl Med Mol Imaging 45(3): 471-495.
- Mehta AR, Armstrong AJ (2016) Tasquinimod in the treatment of castrate-resistant prostate cancer - current status and future prospects. Ther Adv Urol 8(1): 9-18.
- Zhang X, Liu C, Li K, Wang K, Zhang Q, et al. (2019) Meta-analysis of efficacy and safety of custirsen in patients with metastatic castration-resistant prostate cancer. Medicine 98(6).
- Eigl BJ, Chi K, Tu D, Hotte SJ, Winquist E, et al. (2018) A randomized phase II study of pelareorep and docetaxel or docetaxel alone in men with metastatic castration resistant prostate cancer: CCTG study IND 209. Oncotarget 9(8): 8155-8164.
- Harris E (2018) Immunotherapeutics for the treatment of prostate cancer: a patent landscape based on key therapeutic mechanisms of actions. Pharmaceutical patent analyst 7(1): 47- 57.
- Ciccarese C, Massari F, Iacovelli R, Fiorentino M, Montironi R, et al. (2017) Prostate cancer heterogeneity: Discovering novel molecular targets for therapy. Cancer Treat Rev 54: 68-73.
- Hussain M, Daignault-Newton S, Twardowski PW, Albany C, Stein MN, et al. (2018) Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results from NCI 9012. J Clin Oncol 36(10): 991-999.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...