Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Case Report(ISSN: 2641-1687) 
Endoscopic Ultrasound-Guided Fine Needle Aspiration of Small Renal Masses in Selected Patients: Case Reports Volume 2 - Issue 5
De Fabritiis Marco1*, Gurioli Christian2, Mosconi Giovanni1, Dubini Alessandra4, Poletti Venerino2,3 and Ravaglia Claudia2
- 1Department of Nephrology and Dialysis, Morgagni Hospital, Italy
- 2Department Diseases of the Thorax, Morgagni Hospital, Italy
- 3Department of Respiratory Diseases & Allergy, Aarhus University Hospital, Denmark
- 4Department of Pathology, Morgagni Hospital, Italy
Received: June 03, 2020; Published: July 10, 2020
Corresponding author: De Fabritiis Marco, Department of Nephrology and Dialysis, Morgagni Hospital, Forlì, Italy
DOI: 10.32474/JUNS.2020.02.000149
Abstract
The incidental detection of small renal tumors is increasing. Over 75% of such renal tumors, smaller than 4 cm are malignant. Therefore the use of renal biopsy increased during the last decades. It is well tolerated and has a high diagnostic yield. Endoscopic ultrasound-guided fine needle aspiration represents a new option for renal tissue sampling, especially in those patients who simultaneously present suspected neoplastic lesions both in the kidney and in other anatomical districts such as lungs. We present two cases with concomitant intrathoracic and left kidney lesion, in which sampling of renal nodule by EUS-FNA changed the therapeutic management.
Keywords: Renal mass biopsy; EUS-FNA; Mediastinal adenopathy; Lung cancer
Background
More than 13,000 patients are diagnosed with kidney cancer
each year in Italy and more than 3.000 succumb to this disease [1].
Yet, approximately 20% of all surgically resected renal tumors are
benign [2]. Indeed, renal cancer is a heterogeneous disease with
biological behavior ranging from indolent to an aggressive disease.
The incidental detection of small renal tumors is rising because
of the increased use of imaging techniques such as computed
tomography (CT) and/or ultrasonography performed for unrelated
indications [3]. Over 75% of these renal tumors, which are smaller
than 4 cm [small renal masses (SRM)] are malignant. In most of
cases, no specific radiographic features may predict the biological
behavior or even histopathology [4]. The use of renal mass biopsy
(RMB) has increased over the last decade [5]. This procedure is well
tolerated and has a high diagnostic yield and specificity.
Traditionally, tissue diagnosis of renal tumors can be performed
under image guidance (ultrasound, computerized tomography,
or magnetic resonance imaging) with a percutaneous approach.
In general, two different procedures for tissue sampling of renal
masses for histopathological assessment are available: fineneedle
aspiration (FNA) or core biopsy (CB)[6]. FNA is performed
with a thin (23-25 gauge) needle which is inserted into the mass
to remove cells without preserving the histological architecture
of tissue cells. CB is needle core biopsy that samples a portion of
the abnormal tissue. Compared to FNA, core needle biopsies have
a higher diagnostic rate and allow a more accurate histological
examination which could have a prognostic value for example
providing the distinction between invasive and in-situ cancer [7].
In recent years a third option for renal tissue sampling has been
described, represented by Endoscopic ultrasound-guided fine
needle aspiration (EUS-FNA) [8,9]. Endoscopic ultrasound (EUS) is a
combination of endoscopy and ultrasonography. EUS can be used to
visualize and sample mass lesions of lung, mediastinum, pancreas,
gastrointestinal tract, left adrenal gland and retroperitoneum. We present two cases with concomitant intrathoracic and left kidney
lesion, in which sampling of renal nodule by EUS-FNA changed the
therapeutic management. In one of them EUS-FNA was the unique
invasive procedure performed allowing the sampling of both
mediastinal and renal lesions.
Ase Reports
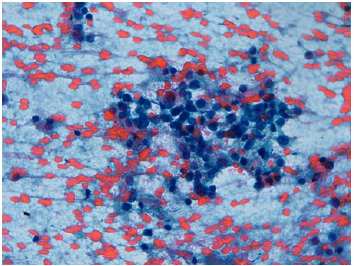
Figure 2: Mediastinal metastasis of carcinoid. High power view of malignant cells with medium sized, dispersed chromatin and fine granular cytoplasm. Positive to immunohistochemical marker with CD 56.
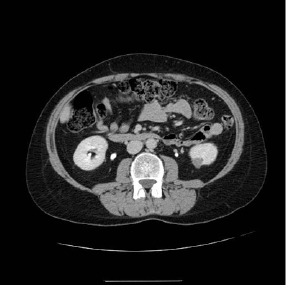
A 42 year old female patient with history of Basedow’s disease treated with methimazole was submitted to right lower lobectomy, one year before, for peripheral pulmonary carcinoid (T1bN1M0 staging), associated to hypercortisolism. After surgery, the ACTH and cortisol values normalized only for a few months. The patient returned to our observation after the execution of positron emission tomography (PET) scan that revealed two hypermetabolic areas (subcarinal lymph node, left pulmonary hilar lymph node); subsequent abdominal and chest computed tomography (CT) scan was performed that showed mediastinal lymphadenopathies of 2cm of smaller diameter and hypodense oval nodule of 15mm of diameter, in the lower polar area of the left kidney (Figure 1). The patient was treated for 3 months with octreotide for the recurrence of the cushing-like symptoms and then was referred for a transesophageal ultrasound (US) that revealed hypoechoic subcarinal lymphadenopathy and a small hypoechoic area within the parenchyma of the left kidney. EUS-FNA of both lesions was performed. Histological and immunohistochemical features were diagnostic of mediastinal metastasis of carcinoid (Figure 2) and clear cell renal carcinoma (Figure 3) respectively.
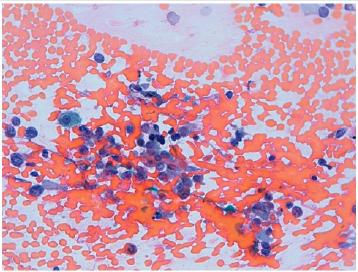
Figure 3: Clear cell renal carcinoma. High power view of clear cell carcinoma cells with polymorphic nuclei.
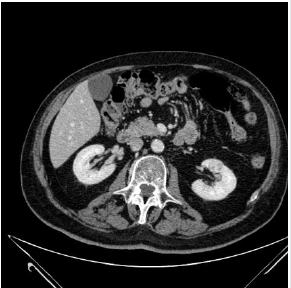
A 74 year old male patient with history of smoking (80 packs of cigarettes per year with a 4-year history of urothelial carcinoma treated with mitomycin for 1 year, was admitted to our Hospital for recurrent haemoptysis. He underwent total body CT that revealed left perihilar pulmonary necrotic lesion, two brain lesions and another lesion in middle-lower zone of the left kidney (Figure 4). The patient was submitted to transesophageal ultrasound that allowed FNA of the nodule of the left kidney. No other lesions were founded at echoendoscopic examination. Bronchoscopy, performed at the same time of EUS, revealed compressive stenosis of the apicodorsal branch of the left upper lobar bronchus. Endobronchial ultrasound at that level showed a large hypoechoic mass on which trasbronchial lung biopsies were carried out. Cytological and histological examination were diagnostic of renal oncocytoma (Figure 5) and pulmonary adenocarcinoma (Figure 6) respectively.
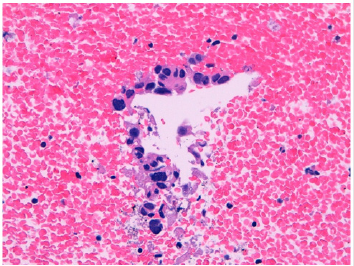
Figure 5: Renal oncocytoma. High power view of small aggregate of medium sized cells, with abundant granular eosinophilic cytoplasm and small regular nuclei, negative to immunohistochemical marker with cytocheratin 7, positive with PAX-8 and CD-117.
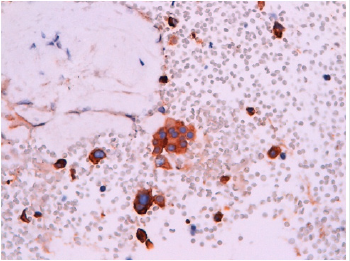
Figure 6: Pulmonary adenocarcinoma. High power view of aggregates of medium to large size malignant cells, with scanty cytoplasm and large and irregular nuclei.
Discussion
Renal tumors are diagnosed through mini-invasive approaches with an increasing frequency. Management of these lesions depends on several factors including biological profile, the presence or absence of specific symptoms and patient comorbidities [10,11]. Tissue sampling of these lesions is traditionally performed by using percutaneous ultrasonographic or CT guidance [12]. According to the European Association of Urology guidelines percutaneous RMB is recommended for active surveillance in selected patients with SRM, before ablative treatments and for patients suffering from renal metastatic disease before starting systemic treatment. For large renal masses scheduled for nephrectomy, RMB is not recommended. The American Urological Association guidelines consider a biopsy when a renal mass is suspected to be metastatic, inflammatory, hematologic, or infectious. RMB appears a safe and feasible technique and should be considered when results would affect patient management [8]. RMB is characterized by high sensitivity and specificity for identifications of malignant tissue (97.5-99.7 and 96.2-99.1%, respectively) [13] with accuracy of 97.1% for malignancy identification [14]. Hematoma is the most common adverse event, observed in up to 91% of cases. Tumor seeding after RMB has a reported rate of less than 0.01% [15].
Because percutaneous biopsy of renal masses is an established procedure that is performed with the patient receiving only local anesthesia and without endoscopy, it seems unlikely that renal biopsy will soon become a routine single indication for EUS. EUS might be attractive to evaluate and possibly allow biopsies in different anatomical sites during a single procedure. In these case reports EUS-FNA allowed to differentiate two different tumors by a single procedure, changing prognosis and therapeutical strategy. In the first case it was necessary to combine a surgical approach for clear cell renal carcinoma with chemotherapy for carcinoid; in the second case a conservative approach was possible for oncocytoma combined with chemotherapy for pulmonary adenocarcinoma. EUS approach is however easily feasible only when the left kidney is involved, because it may visualized when the echoendoscope is located inside the stomach.
Conclusion
The clinical applications of EUS-FNA continue to expand and can be useful in the diagnosis of SRM. EUS allows examination and biopsy of masses or lesions within the kidney, thus facilitating the diagnosis of early RCC. This technique should be considered when concomitant lesions are founded in different anatomical sites and whose characterization would affect patient management, changing prognosis and therapeutical strategy.
Author Contribution
Manuscript preparation, data collection and analysis: De Fabritiis M., Gurioli C.; lab assay and data collection: Dubini A.; Ravaglia C.; main conceptualization and supervision: De Fabritiis M, Poletti V.
Funding
These case reports received no grant from any fundingn agency in the public, commercial or not-for-profit sectors.
Conflicts of Interest
The Autors declare no conflit of interest.
References
- AIOM-Airtum I numeri del cancro in Italia.
- Johnson MH, Sozio SM, Sharma R, Iyoha E, Bass EB, et al. (2016) Improving needle biopsy accuracy in small renal mass using tumor-specific DNA methylation markers. Cancer.
- Znaor A, Lortet-Tieulent J, Laversanne M (2015) International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 67(3): 519-530.
- Kane CJ, Mallin K, Ritchey J (2008) Renal cell cancer stage migration: analysis of the national cancer data base. Cancer 113(1): 78-83.
- Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, et al. (2008) Renal mass biopsy a renaissance? J Urol 179(1): 20-2
- Tsivian M, Rampersaud EN Jr, del Pilar Laguna Pes M (2014) Small renal mass biopsy–how, what and when: report from an international consensus panel. BJU Int 113(6): 854-863.
- Moschetta M, Telegrafo M, Carluccio DA (2014) Comparison between fine needle aspiration cytology (FNAC) and core needle biopsy (CNB) in the diagnosis of breast lesions. G Chir 35(7-8): 171-176.
- DeWitt J,Gress FG, Levy MJ, Hernandez LV, Eloubeidi MA, et al. (2009) EUS-guided FNA aspiration of kidney masses: a multicenter U.S. Experience. Gastrointestinal Endoscopy 70(3): 573-578.
- Everson LA, Iglesias Lopes R, Kumar A, Marmo Lucon A, Dall’oglio M, et al. (2008) Endoscopic Ultrasound Facilitates Histological Diagnosis of Renal Cell Cancer. Journal of Endo urology 22: 2447-2450.
- Ramı´rez ML (2007) Evans CP. Current management of small renal masses. Can J Urol 14(Suppl 1): 39-47.
- Silverman SG, Israel GM, Herts BR (2008) Management of the incidental renal mass. Radiology 249(1): 16-31.
- Johnson PT, Nazarian LN, Feld RI (2001) Sonographically guided renal mass biopsy. J Ultrasound Med 20(7): 749-753.
- Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, et al. (2016) Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 69(4):660-6
- Jeon HG, Seo SI, Jeong BC, Jeon SS, Lee HM, et al. (2016) Percutaneous kidney biopsy for a small renal mass: a critical appraisal of results. J Urol 195(3):568-5
- Viswanathan A, Ingimarsson JP, Seigne JD, Schned AR (2015) A singlecentre experience with tumour tract seeding associated with needle, manipulation of renal cell Carcinomas. Can Urol Assoc J 9(11-12): 890-893.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...