Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2643-6760
Case Report(ISSN: 2643-6760) 
Recurrent Hypertrophic Pyloric Stenosis in a Male Child: A Case Rarity Volume 5 - Issue 3
Nitin Jain1, Parveen Kumar2* and Shandip Sinha3
- 1Department of Pediatric Surgery, RMC, Bareilly, India
- 2Department of Pediatric Surgery, Chacha Nehru Bal Chikitsalya, India
- 3Department of Pediatric Surgery, Madhukar Rainbow Children Hospital, India
Received:June 10, 2020; Published: July 07, 2020
Corresponding author: Parveen Kumar, Assistant Professor, Department of Paediatric Surgery, Chacha Nehru Bal Chikitsalya, New Delhi, India
DOI: 10.32474/SCSOAJ.2020.05.000211
Abstract
Infantile hypertrophic pyloric stenosis (IHPS) is the most frequent surgical condition in children. Incomplete pyloromyotomy is an infrequent complication, but recurrent pyloric stenosis (RPS) is extremely rare. We report here a case of late-onset recurrence of IHPS in a 7-year-old male child, after successful pyloromyotomy. He presented with abdominal pain, recurrent episodes of nonbilious vomiting and upper abdominal fullness after taking meals that relieved on vomiting. These symptoms started around 4 months after successful pyloromyotomy done at 4 weeks of age. Child underwent series of radiological investigations including upper GI endoscopy that confirmed gastric outlet obstruction with thickened pylorus. Multiple management options are proposed but we preferred ‘heineke mikulicz pyloroplasty’ in our case.
Introduction
Hypertrophic pyloric stenosis (HPS) is a common cause of
gastric outlet obstruction (GOO) in neonates, with an incidence of
1.5 to 3 per 1000 live births. Onset is seen usually between 2 to 8
weeks of life, with classic presentation of feeding intolerance with
projectile, non-bilious emesis. Diagnostic work up usually reveals
a hypokalemic, hypochloremic metabolic alkalosis. Ultrasound or
fluoroscopic upper gastrointestinal series can be used to facilitate
diagnosis when the clinical picture and physical exam are equivocal.
The etiology of IHPS is not properly understood. Various authors
have suggested its congenital or acquired nature [1]. Ramstedt’s
pyloromyotomy is the current standard of care, with high curative
rate and minimal post-operative morbidity. As incomplete
pyloromyotomy is a well-documented post-operative complication,
there are very few cases of true recurrent PS.
Multiple options are proposed for the management of recurrent
PS including pneumatic dilatation or botulinum toxin injection as
well as operative management in form of pyloroplasty, Billroth I,
Billroth II procedures [2-4]. Here, we present a case of isolated
recurrent PS in 7 years old male child following successful
management with routine pyloromyotomy.
Case Report
A 7-year-old male child presented with abdominal pain, recurrent episodes of non-bilious vomiting and upper abdominal fullness after taking meals that relieved on vomiting. Parents also gave history of vomiting of partially digested food material eaten few hours earlier. These symptoms started around 4 months after the child had undergone successful pyloromyotomy at 4 weeks of age. At the time of first presentation during neonatal period, symptoms of repeated episodes of vomiting and upper abdominal fullness immediately after feeding were recognised by parents and child underwent open Ramstedt pyloromyotomy. At home, he tolerated full feeds with no vomiting and gained weight. Child was symptoms free until about 4 months postoperatively. Initially the symptoms were not severe and child had bloating, early satiety, foul-smelling regurgitations and over the following year, child experienced few episodes of infrequent non-bilious vomiting. As per his mother child was gaining weight at good pace until when the frequency of vomitings gradually increased over 4-5 years. Child later developed abdominal pain and recurrent non bilious vomitings after about 20 to 30 minutes of a feed.
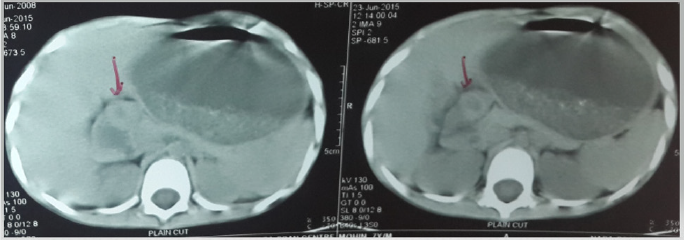
During follow-up child was being treated for gastro-esophageal reflux with persistent symptoms. Later, child presented to our emergency. On examination, he had signs of dehydration, with pulse rate of 119/minute, low volume and B.P was 92/48 mmHg. Blood investigations revealed Hb 9.3g/dl, TLC 7250/dl, Platelet count 3.59L/mm3, HCT 41%, protein 4.8g/dl, serum albumin 2.3g/dl, blood urea 21 mg/dl, serum creatinine 0.9mg/dl, Na+ 129meq/L, K+ 3.1meq/L & Cl- 95meq/L. Venous blood gas (VBG) analysis showed pH 7.47, pCo2 34, pO2 59, Hco3 29. Weight of the child at presentation was less than 3rd Centile on growth chart. Child was managed with fluid resuscitation, and blood investigation revealed correction of dehydration as manifested by normalisation of hematocrit to 36% and VBG pH 7.36, pCo2 31, pO2 66, Hco3 24. Child underwent series of radiological investigations that included USG abdomen, which showed dilated stomach and contrast enhanced CT scan with oral contrast confirmed grossly dilated stomach extending inferiorly to the level of iliac fossa and displaced the bowel loops posteriorly. On successive sections there was circumferential thickening of distal stomach. All these features pointed towards GOO because of PS (Figure 1).
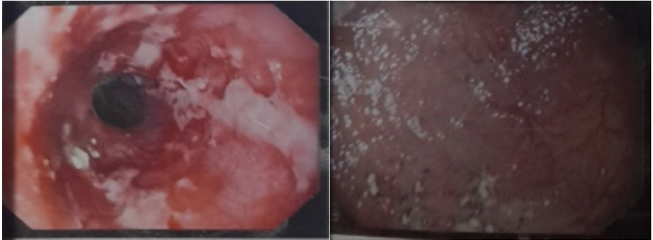
Upper GI endoscopy showed multiple ulcerations with friability at the lower end of esophagus. Stomach was dilated with food residue and normal fundus, body and antral regions but pyloric opening couldn’t be negotiated. After workup child was planned for surgery. Intra-operative findings were dilated stomach and thickened pylorus. We performed ‘heineke mikulicz pyloroplasty’ involving transversely closed longitudinal incision across the pylorus. The nasogastric tube was removed on 3rd post-operative day (POD) and child was allowed liquids on 4th POD with soft diet on 5th POD (Figure 2).
Outcome and follow-up
He was discharged on POD-7 on soft diet and tab Lanzol 15mg DT for 2 weeks. At his 2-week post-operative visit, he was asymptomatic and tolerated feedings without bloating or emesis. The histopathological examination was consistent with HPS. He remained completely asymptomatic at 1-year follow-up.
Discussion
Ramstedt’s pyloromyotomy (PM) remains the gold standard treatment for IHPS [5]. Emesis is a frequent postoperative complaint after pyloromyotomy and it occurs in 36–90%of cases [4]. Usually vomiting stops spontaneously after few days. Incomplete pyloromyotomy is suspected when vomiting persists and lasts of more than 5 days. There is a criterion to differentiate recurrent PS from incomplete pyloromyotomy which includes [6];
a. The patient must have resolution of symptoms for duration of
at least three weeks postoperatively.
b. The patient must demonstrate weight gain.
c. Evidence of restenosis must be confirmed with imaging or on
exploration (all these conditions are consistent with our case).
Huang et al. [7] showed that the immediate postoperative
resolution of emesis after a successful pyloromyotomy is from
an increase in the diameter of the intramuscular pyloric canal
rather than an immediate regression in the thickness or length of
the pyloric muscle. In incomplete pyloromyotomy pyloric canal
diameter will not be seen to increase in diameter on USG who
presented with repeated vomiting in immediate post-operative
period. True recurrence of HPS is quite rare with only a few case
reports in literature.
The etiology of recurrent PS, as IHPS itself, is unclear. It appears
that pathological evolution of HPS as well as recurrent PS as a true
surgical entity is similar and separate from incomplete PM. It has
been suggested that the process which drives hypertrophy of the
pylorus is in still in evolution when initial pyloromyotomy was
performed which after initial symptom free interval progressed
to true recurrent PS [3,8]. In any case of recurrent PS, initial
management is conservative with bowel rest and nasogastric
decompression with trial periods ranging from 7 to 21 days. The
medical management using atropine, botulinum toxin injection and
surgical management including pyloroplasty, Billroth I, Billroth II
procedures, and pneumatic balloon dilatation has been proposed
[2-4]. We did ‘heineke mikulicz pyloroplasty’ in our case because of
its ease and simplicity with preservation of normal anatomical tract
and less complications.
A close differential diagnosis of Jodhpur disease (JD) needs
mention. It presents as primary acquired gastric outlet obstruction
in infancy and childhood, with very similar presentation to our
case. Jodhpur disease has predilection for male sex [9]. There
are certain differentiating features between these two entities
[10]. A) Ultrasonography shows normal pyloric canal with no
pyloric muscle hypertrophy in Jodhpur disease (JD) but shows
narrow pyloric canal with pyloric muscle hypertrophy in HPS. B)
UGIE in JD shows no intra-luminal pathology with normal gastric
mucosal rugosities in a large-sized stomach while in HPS Antral
folds hypertrophy with dilated stomach. C) On histopathological
examination (HPE), Jodhpur disease shows normal cellular pattern of all coats without inflammatory and fibro-proliferative nature
[9]. HPE was consistent with HPS in our case. It showed marked
congestion of pyloric mucosa. Muscularis mucosa and muscularis
propria showed hypertrophy and hyperplasia of muscle bundles
along with haphazardly directed muscle bundles with interspersed
fibro-collagenous tissue.
Conclusion
True Recurrent PS is a rare condition with unclear etiology. There may be an increasing incidence due to early diagnosis with modern investigations. Utilizing the evidences & criteria available for diagnosis, we believe pyloroplasty seems to be the most effective and safest intervention for recurrent PS.
Conflicting Interest
Nil
Acknowledgement
Patient was managed at Maulana Azad Medical college, Lok Nayak Hospital.
References
- Al-Ansari A, Altokhais TI (2016) Recurrent pyloric stenosis. Pediatr Int Off J Jpn Pediatr Soc 58(7): 619-621.
- Feng J, Gu W, Li M, Yuan J, Weng Y, et al. (2005) Rare causes of gastric outlet obstruction in children. Pediatr Surg Int 21: 635-640.
- Ankermann T, Engler S, Partsch CJ (2002) Repyloromyotomy for recurrent infantile hypertrophic pyloric stenosis after successful first pyloromyotomy. J Pediatr Surg 37(11): E40.
- Boybeyi Ö, Karnak İ, Ekinci S, Ciftci AO, Akçören Z, et al. (2010) Late-onset hypertrophic pyloric stenosis: definition of diagnostic criteria and algorithm for the management. J Pediatr Surg 45: 1777-1783.
- Keys C, Johnson C, Teague W, Mackinlay G (2015) One hundred years of pyloric stenosis in the Royal Hospital for Sick Children Edinburgh. J Pediatr Surg 50: 280-284.
- Cappiello CD, Strauch E (2014) A rare case of recurrent hypertrophic pyloric stenosis. J Pediatr Surg Case Rep 2: 519-521.
- Huang Y, Lee H, Yeung C, Chen W, Jiang C, et al. (2009) Sonogram before and after pyloromyotomy: the pyloric ratio in infantile hypertrophic pyloric stenosis. Pediatr Neonatol 50: 117-120.
- Kuckelmana J, Marencoa C, Doa W, Barlowb M (2019) Recurrent pyloric stenosis and definitive operative management with repeat pyloromyotomy. J Pediatr Surg Case Rep 42: 24-27.
- Sharma KK, Ranka P, Goyal P, Dabi DR (2008) Gastric outlet obstruction in children: an overview with report of “Jodhpur disease” and Sharma’s classification. J Pediatr Surg 43: 1891-1897.
- Kajal P, Bhutani N, Kadian YS (2019) Primary acquired gastric outlet obstruction (Jodhpur disease). J Pediatr Surg 40: 6-9.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...