Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2643-6760
Research Article(ISSN: 2643-6760) 
RADA16, a Self-Assembling Peptide (Sap) For Haemostasis in Nasal Endoscopic Surgery, a Case Series in Australia Volume 6 - Issue 5
Dilshard Soodin1, Sheldon Chong2, David Morrissey3, Jennifer Ha4, Yuresh Naidoo5, Andrew Foreman6, Michael Switajewski7, Dan Robinson8, Alisson Sprague9 and Maurice Bagot D’arc10*
- 1ENT consultant, Norwest Hospital, Sydney, NSW, Australia
- 2ENT consultant, Calvary Central Hospital, Adelaide, SA, Australia
- 3ENT consultant, St Vincent Hospital, Toowoomba, QLD, Australia
- 4ENT consultant, St John of God Hospital, Perth, WA, Australia
- 5Associate Professor, University of Sydney, NSW, Australia
- 6ENT consultant, Memorial Hospital, Adelaide, SA, Australia
- 7ENT consultant, Memorial Hospital, Adelaide, SA, Australia
- 8ENT consultant, Pindara Hospital, Gold Coast, QLD, Australia
- 9ENT consultant, Pindara Private, Benowa, QLD, Australia
- *10Retired ENT surgeon, BluePharm SAS, rue Davioud, France
Received:May 2, 2022; Published:May 11, 2022
Corresponding author: Maurice Bagot D’arc, Retired ENT surgeon, BluePharm SAS, rue Davioud 17, 75016 Paris, France
DOI: 10.32474/SCSOAJ.2022.06.000247
Abstract
Background: Assess safety, efficacy, and ease of use of a novel haemostatic agent in various procedures in endonasal surgery.
Methods: Prospective multicenter case series. One hundred and sixty-seven consecutive patients undergoing endonasal surgery including turbinate reduction, septoplasty and FESS procedures received RADA16 2.5% PuraStat™, a novel synthetic peptide solution at the end of surgery to secure haemostasis. Ease of use and efficacy were assessed intraoperatively and at follow up.
Results: PuraStat™ was found to be ready to use, easy to apply and fast to achieve haemostasis. The transparency of the product makes its application to bleeding surfaces easier than alternative bulky and opaque products. It appears to be as effective for haemostasis as other endonasal products with haemostasis efficacy of 98.2%. The use of PuraStat™ mainly in the absence of nasal packing and minimal diathermy was also marked by a low incidence of adhesions requiring additional treatment (4.2%) with minimal crusting.
Conclusion: RADA16 2.5% is a novel haemostatic peptide easy to use and to apply to bleeding nasal mucosa. Further comparative studies are needed to assess long-term efficacy and safety in performing hemostasis.
Keywords: RADA16; PuraStat; Case Series; Endonasal Surgery; Self-assembling Peptides; Haemostasis; Turbinoplasty; Turbinectomy; FESS
Introduction
Endoscopic Endonasal Surgery (EES) has seen rapid progress in both instrumentation and visualization allowing surgeons unprecedented access to the sinonasal cavity. In this surgical field, bleeding is a common intraoperative issue limiting visualization. It is also an important post-operative complication. Typically, bleeding is controlled at the end of the procedure by diathermy, application of topical haemostatic agents or nasal packing. It is acknowledged that excessive use of cautery may facilitate crusting and adhesion formation. Post-operative bleeding can lead to significant discomfort and concern for the patient and may ultimately lead to a return to theatre. Hence, surgeons regularly use nasal packing in the immediate post-operative period to minimize bleeding, however it compromises patient comfort. Resorbable materials and topical haemostats may be useful to avoid packing. The ideal, topical haemostat suitable for endonasal surgery should be endoscopically applicable, conform to narrow spaces, have a rapid onset of action and no risk of migration. It should allow avoidance of nasal packing while preventing postoperative bleeding and supporting wound healing. Such an agent should result in minimal crusting and adhesion formation.
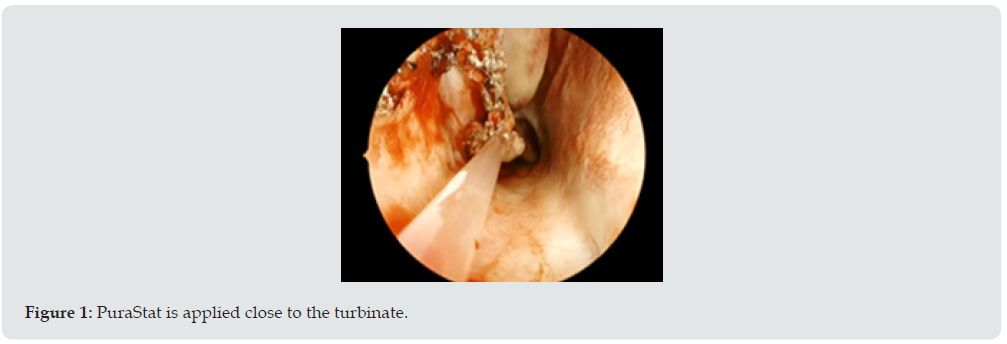
RADA16 2.5% PuraStat™ (3-D Matrix Europe, Caluire-et-Cuire, France), is a fully synthetic medical device, CE-marked and licensed for topical hemostasis in Australia. It is indicated for haemostasis of oozing bleeding from parenchyma and surrounding tissues of solid organs, vascular anastomoses and small blood vessels or capillaries of the GI tract encountered during surgery when haemostasis by ligation or standard means is insufficient or impractical [1]. It comes in a pre-filled sterile syringe (available in 3 and 5mL) as a viscous transparent 2.5% solution containing a single peptide RADA16, which relies on the capacity of the repeating sequences of peptide to self-assemble in presence of blood or tissue and form a biocompatible and bioresorbable hydrogel. It is stored at temperatures between 2°C and 8°C and does not require any preparation. Contact between the product and a liquid with a physiological pH such as blood causes the acidic peptide solution to be neutralized resulting in the quick formation of a hydrogel [2]. The hydrogel quickly coats the point of bleeding forming a physical barrier over the bleeding vessel and causes coagulation in the adjacent vascular wall resulting in haemostasis. The colourless product is applied right at the bleeding point at the end of the procedure via the thin nozzle delivered with the product. There is no need for compression. RADA16 2.5% has been shown to stop bleeding in less than 15 seconds [3], independently of the patient’s coagulation capability while maintaining a clear visualization of the treated surfaces (Figure 1). It is suitable for endoscopic use and is biocompatible and bioresorbable within approximately 30 days via enzymatic action. In addition, the product has no swelling capacity and may be used in the vicinity of fragile structures [1].
Methods
A prospective multicenter case series was designed to assess the clinical performance in haemostasis and safety of RADA16 2.5% in patients undergoing endoscopic endonasal surgery in Australia. Patients were required to follow up between 2 and 5 weeks after surgery. The primary objective was to assess the effectiveness of RADA16 2.5% in stopping intraoperative nasal bleeding. Secondary objectives included the incidence of delayed bleeding at the followup visit, the level of crust formation, the rate of adhesion formation, and the incidence of local infection. This was determined through endoscopic observation of the nasal mucosa. Safety aspects formed other secondary endpoints. The inclusion criteria encompassed all consecutive patients undergoing endoscopic endonasal elective surgery, where the intraoperative use of a haemostat was deemed necessary. This prospective observational study was performed in compliance with the Helsinki declaration and approved by local ethical committees. Patients consented to the use of haemostatic agents such as RADA16 and consented for their deidentified data being included in the case series as part of their consent for endonasal surgery.
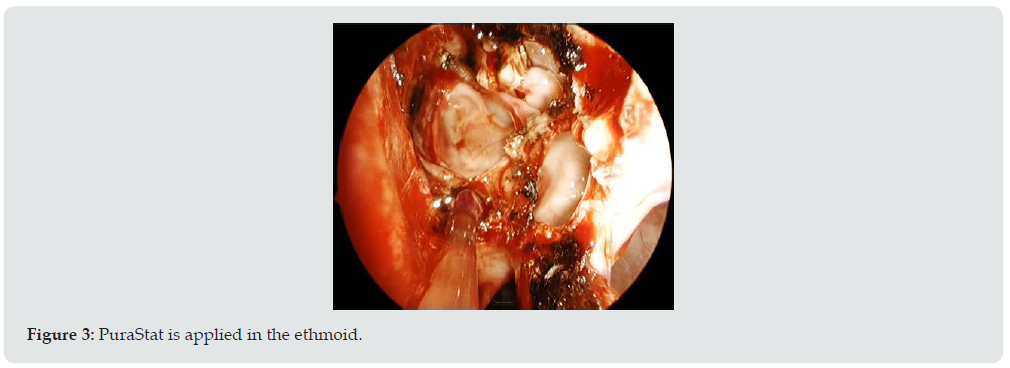
Patients enrolled underwent either a turbinate reduction surgery alone or combined with a FESS (Functional Endoscopic Sinus Surgery) procedure such as maxillary antrostomies and ethmoidectomies and with or without septoplasty. The aim of this study was to evaluate the feasibility and efficacy of the product in real life conditions via a questionnaire developed to gather feedback on its use in various scenarios. The surgeons and scrub staff received training on the product prior to use. Surgeons were asked to score several items such as, overall performance and handling, ready-touse benefit, transparency benefit, effectiveness of haemostasis and overall satisfaction on a 5point scale from 1 (fair) to 5 (excellent) and to comment on their overall satisfaction. Efficacy was assessed by surgeon reporting of any bleeding occurring intra-operatively or up to 24 hours of the surgery and requiring treatment. Delayed bleeding was assessed during the follow-up visit by reporting of any bleeding episode occurring from 24 hours after the surgery and requiring additional treatment. Safety was established by reporting of adverse events during patient hospitalization and at the follow-up visit. Anonymized data were collected and analyzed using descriptive statistics for quantitative variables and qualitative variables (Figures 2 &3).
Results
Between September 2017 and March 2019, 167 patients were recruited by 11 senior surgeons and underwent ESS using mucosalsparing techniques, with meticulous haemostasis during and at the end of the procedure. One hundred and forty-eight patients had a turbinate reduction surgery alone or combined with other procedures, such as a Functional Endoscopic Sinus Surgery with or without septoplasty. Nineteen patients experienced a FESS with or without septoplasty but in absence of turbinate reduction. Details of the types of procedures is given in Table 1. There was minimal use of diathermy confirmed in at least 144 cases (86%) and 131 patients (78%) did not receive nasal packing. 3ml and 5ml syringes of PuraStat™ were used with a mean dose of 3.12ml in total for both sides of the nose.
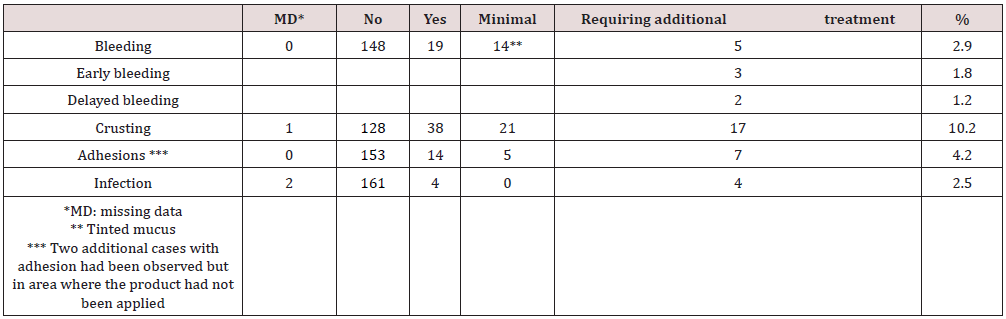
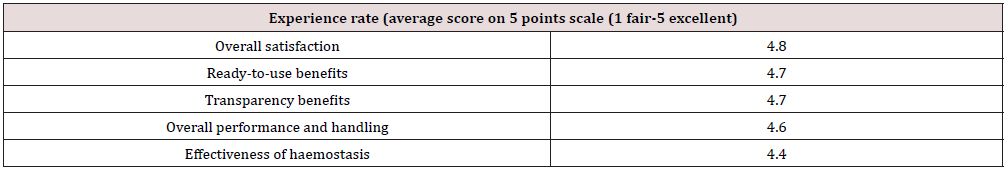
Table 2 presents the mean scores given by surgeons relating to their experience using the device. PuraStat scored highly on items such as overall satisfaction (4.8 out of 5) and ready to use benefit (4.7). Visualization of the operative surface through PuraStat was recognized as a real benefit (score of 4.7). The overall performance and handling scored 4.6 and the effectiveness of haemostasis scored 4.4. On average, the follow up visit occurred 13 days after surgery (ranges 1-49). All patients performed nasal irrigation with saline approximately 3 to 4 times per day. One hundred and forty-eight patients did not report any post-operative bleeding. 14 reported minor bleeding (mainly marked by tinted mucus on the bolster), that resolved spontaneously. of these 14 patients, 3 were on anticoagulant medication and one had a Von Willebrand Disease. Five patients (2,9%) experienced a bleeding that required additional treatment. These patients had all experienced turbinate reductions with septoplasty and FESS and had not received nasal packing at the end of the procedure. Three of them (1.8%) bled the same day of surgery; among them 1 was treated with tranexamic acid and 2 had nasal packing. The 2 other patients (1.2%) had delayed bleeding, one at day 7 from the sphenoid sinus where RADA16 had not been applied and the other one at day 11 from the FESS cavity. The latest patient was subsequently diagnosed with myelodysplasia. Those 2 patients were also treated locally, one with tranexamic acid and one with packing. None of the 5 bleeding patients had to return to theatre. So, the 3 patients who had post-operative bleeding on the day of surgery were considered as product failure, resulting in a haemostatic efficacy of 98.2% (164/167). Crusting was absent or minimal in 149 patients (89.2%) and noticeable in 17 (10.2%). Surgeons often commented this was expected. Adhesions were not observed in 153 cases (91.6%) and were present in 14 cases (8.38%) at the first follow up visit. In 5 cases, adhesions were minor and easy to divide and in 9 cases more significant, of which 2 were excluded from the analysis. as they did not develop in areas where PuraStat has been applied. So, the rate of adhesions formed in areas where RADA16 was applied, and which required treatment was 4.2% (7/167). Four infections were observed which were not considered as product related. No other complications were reported. No adverse event was reported (Table 3).
Table 2: Evaluation of feasibility and effectiveness of RADA16 2.5% PuraStat in stopping intraoperative bleeding (average score).
Discussion
A major challenge in endonasal surgery is the narrow operative space. After endonasal surgery there is typically mucosal edema, exposed mucosal edges and a consequent risk of bleeding and adhesion formation. In addition, sinus surgery bears additional risk like CSF leakage or orbital haematoma. Excessive perioperative bleeding has been reported to occur in approximately 5% of patients after sinonasal surgery, with less than 1% requiring transfusion [4]. In our study the rate of patients with a bleeding requiring additional treatment is 2.9%. Stankiewicz in a large retrospective study of 3,402 patients over 25 years, al. (2011) only considered bleeding as a complication when it necessitated packing or surgery for control [5]. Thus, according to this definition, as only 3 patients were packed and none had to return to theatre, the haemostatic effectiveness when considering patients who didn’t experience postoperative bleeding or those who bled but didn’t require packing was 98.2% (164/167). This bleeding rate of 2.9% compares favorably with other series with the published bleeding rates reported from 2% up to 3.8% in similar populations [6,7].
However direct comparisons are not possible because the population in our study is heterogeneous and because a high proportion of patients in the literature received nasal packing while it was only a minority in our study. Multiple haemostatics and packing options are marketed to help control post-operative haemostasis and minimize adhesion formation. Absorbable nasal packing materials have some patient comfort advantages but may still require removal due to slow breakdown. Several currently available agents have demonstrated satisfactory haemostatic properties. However, they have the risk of potential transmission of blood borne diseases as they contain human plasma derivatives like thrombin. They have also been reported to result in adhesion formation, which remains a major concern occurring in 10 to 36% of cases [6,8-10]. In our study, 14 patients were found with adhesion at the first follow up visit (8.38%). However, the rate of adhesions requiring additional treatment formed in areas where PuraStat was applied was 4.2% (7/167). This adhesion rate is low in comparison to the literature. Crusting was sometimes observed in accordance with the surgeons’ expectations but was easy to remove. There were 4 postoperative infections observed in that cohort, considered as not related to the product, while infection represent the most common cause for readmission (36.3%) [6].
PuraStat was recently licensed in Australia and clinical data on its effectiveness in ENT surgery is limited. Lee and Ananda (2017) published a first series of 60 patients mainly after turbinate reduction surgery [11] and found this product effective in absence of any re-bleeding with limited adhesion formation and adequate healing. This prospective data collection performed in 8 centres by 9 surgeons demonstrates a range of indications for the use of PuraStat. Outcome data related to feasibility, effectiveness and safety has been obtained from users. This has highlighted the ease of use of the device, its efficacy in controlling oozing at the end of the procedure and the benefit of transparency in maintaining a clear view of the operative sites, and no side effect has been reported confirming the high safety profile observed in the literature after PuraStat use. The effectiveness and the safety of PuraStat has been assessed in several studies in gastrointestinal endoscopy for haemostasis after endoscopic tissue resection or to stop primary bleeding [12-18] and for haemostasis on vascular anastomoses [19-21] as in general surgery [22,23] with haemostatic rate ranging from 72.6% to 100% [2]. However, this study has several limitations. It was designed as a first survey to assess the ease of use, and performance during the initial follow up period. The standard follow-up helped to evaluate the product at least during the first two weeks after surgery but only limited data was collected. The cohort remains small and there was no control group, resulting in difficulty making comparisons to other types of haemostatic agents or packing. Surgeries were performed by several surgeons in various centres, but the patient population is quite homogenous. Controlled randomized studies are needed to confirm the efficacy of the product in a longer followup period.
Conclusion
By evaluating the use of this product in 167 consecutive cases, it has been established that PuraStat is easy to use and is shown to be effective in achieving intra-operative hemostasis in a wide variety of surgical scenarios in endonasal surgery with reduced adhesion formation. Further studies are needed to assess long-term efficacy and safety in performing hemostasis.
Authors’ Contribution
Soodin D, Chong S, Morrissey D, Ha J and Naidoo Y, all otolaryngologists contributed by provision of patients, collection and assembly of the data, careful revision of the paper and comments and final approval of the manuscript. The other authors Foreman A, Switajewski A, Robinson D, Sprague A all otolaryngologists contributed by provision of patients, collection and assembly of data, and final approval of the paper. Bagot d’Arc M, retired otolaryngologist developed the case series design, performed the data interpretation and wrote the paper.
Financial Disclosure
Surgeons were compensated by 3-D Matrix, Australia to offset costs associated with usage and feedback provision.
Conflict of Interest
The authors declare conflict of interest related to the company for compensation for the costs associated with the data collection. The last author declares that he is a retired ENT surgeon serving as a consultant for the company.
Acknowledgement
The authors would like to thank Mrs. Claudia Delin for her statistical support.
References
- (2021) PuraStat Instruction For Use, 3D Matrix Medical Technology, Caluire et Cuire, France, IFU-007 Rev 2021 /09.
- Sankar S, O’Neill K, Bagot D’Arc M, Rebeca F, Buffier M, Aleksi E, et al. (2021) Clinical Use of the Self-Assembling Peptide RADA16: A Review of Current and Future Trends in Biomedicine. Front Bioeng Biotechnol 9: 679525.
- Ellis-Benke RG, Liang YX, Tay DKC, Kau PWF, Schneider GE, et al. (2006) Nano hemostat solution: immediate hemostasis at the nanoscale. Nanomedicine 2: 207-215.
- Hopkins C, Browne JP, Slack R, Lund VJ, Topham J, et al. (2006) Complications of Surgery for Nasal Polyposis and Chronic Rhinosinusitis: The Results of a National Audit in England and Wales. The Laryngoscope 116(8): 1494-1499.
- Stankiewicz JA, Lal D, Connor M, Welch K (2011) Complications in endoscopic sinus surgery for chronic rhinosinusitis: a 25‐year experience. The Laryngoscope 121(12): 2684-2701.
- Khoury H, Bellamkonda N, Benharash P, Lee JT, Wang MB, et al. (2021) National Analysis of 30-Day Readmission Following Inpatient Sinus Surgery for Chronic Rhinosinusitis. The Laryngoscope 131(5): E1422-E428.
- Antisdel JL, Meyer A, Comer B, Jang D, Gurrola J, et al. (2016) Product comparison model in otolaryngology: Equivalency analysis of absorbable hemostatic agents after endoscopic sinus surgery. The Laryngoscope 126: S5-S13.
- Valentine R, Wormald PJ (2010) Nasal dressings after endoscopic sinus surgery: what and why? Curr Opin Otolaryngol Head Neck Surg 18: 44-48.
- Athanasiadis T, Beule A, Robinson BH, Robinson SR, Z Shi, et al. (2008) Effects of a Novel Chitosan Gel on Mucosal Wound Healing Following Endoscopic Sinus Surgery in a Sheep Model of Chronic Rhinosinusitis. Laryngoscope 118(6): 1088-1094.
- Chandra RK, Conley DB, Haines GK, Kern RC, et al. (2005) Long-term effects of FloSeal packing after endoscopic sinus surgery. Am J Rhinol 19(3): 240-243.
- Lee MF, Ma Z, Ananda A (2017) A novel haemostatic agent based on self-assembling peptides in the setting of nasal endoscopic surgery, a case series. International Journal of Surgery Case Reports 41: 461-464.
- Yoshida M, Goto N, Kawaguchi M, Koyama H, Kuroda J, et al. (2014) Initial clinical trial of a novel hemostat, TDM-621, in the endoscopic treatments of the gastric tumors. Journal of Gastroenterology and Hepatology 29 (Suppl 4): 77-79.
- Uraoka T, Ochiaï Y, Fujimoto A, Goto O, Kawahara Y, et al. (2016) A novel fully synthetic and self-assembled peptide solution for endoscopic submucosal dissection-induced ulcer in the stomach. Gastrointest Endosc 83(6): 1259-1264.
- Pioche M, Camus M, Rivory J, Leblanc, Lienhart I, et al. (2016) A self-assembling matrix-forming gel can be easily and safely applied to prevent delayed bleeding after endoscopic resections. Endosc Int Open 4(4): E415-E419.
- Subramaniam S, Kandiah K, Thavalesekaran S, Longcroft-Wheaton G, Bhandari P, et al. (2019) Haemostasis and prevention of bleeding related to ER: The role of a novel self-assembling peptide. United European Gastroenterol J. 7(1): 155-162.
- Subramaniam S, Kandiah K, Chedgy F, Fogg C, Thayalasekaran S, et al. (2020) A novel self-assembling peptide for hemostasis during endoscopic submucosal dissection: a randomized controlled trial. Endoscopy 53(1): 27-35.
- de Nucci G, Reati R, Arena I, Bezzio C, Devani M, et al. (2020) Efficacy of a novel self-assembling peptide hemostatic gel as a rescue therapy for refractory acute gastrointestinal bleeding. Endoscopy 52(9): 773-779.
- Branchi F, Klingenberg-Noftz R, Friedrich K, Bürgel N, Daum S, et al. (2021) PuraStat in gastrointestinal bleeding: results of a prospective multicentre observational pilot study. Surg Endosc 36(5): 2954-2961.
- Masuhara H, Fuji T, Watanabe Y, Koyama N, Tokuhiro K (2012) Novel Infectious Agent-Free Hemostatic Material (TDM-621) in Cardiovascular Surgery. Ann Thorac Cardiovasc Surg 18: 444-451.
- Giritharan S, Salhiyyah K, Tsang GM, Sunil K Ohri (2018) Feasibility of a novel, synthetic, self-assembling peptide for suture-line haemostasis in cardiac surgery. J Cardiothorac Surg 13(1): 68.
- Morshuis M, Schönbrodt M, Gummert J (2019) Safety and Performance of a Self-Assembling Peptide Haemostat for the Management of Bleeding after Left Ventricular Assist Device. Implantation: Outcomes of a Post Market Clinical Follow-Up Study. The Journal of Heart and Lung Transplantation 38(4): S194.
- Nahm C, Popescu I, Botea F, Fenwick S, Fondevila C, et al. (2022) A multi-center post-market clinical study to confirm safety and performance of PuraStat® in the management of bleeding during open liver resection. HPB (Oxford) 24(5): 700-707.
- Gangner Y, Bagot d'Arc, Delin C (2022) The use of self-assembling peptides (PuraStat) for hemostasis in cervical endocrine surgery. A real-life case series of 353 patients. Int J Surg Case Rep 94: 107072.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...