
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2643-6760
Research Article(ISSN: 2643-6760) 
Hepatitis B Virus Infection among Resident Physicians and Nurses in Tertiary Hospitals in Sana’a City, Yemen Volume 5 - Issue 5
Abdul Salam Mohamed Al Makdad2, Abdulrahman Y Al-Haifi1, Hassan A Al-Shamahy3, Mohammed Kassim Salah2 and Ammar Hashim Abdullah Obaid3
- 1Department of Microbiology, Faculty of Medicine, Thamar University, Damar, Yemen
- 2Department of of Medicine, Faculty of Medicine, Thamar University, Damar, Yemen
- 3Medical Microbiology and Clinical Immunology Department, Faculty of Medicine and Health Sciences, Sana’a University, Republic of Yemen.
Received: August 28, 2020; Published: September 09, 2020
Corresponding author: Hassan A Al-Shamahy, Faculty of Medicine and Heath Sciences, Sana’a University, Yemen
DOI: 10.32474/SCSOAJ.2020.05.000221
Abstract
Health care workers (HCWs) represent one of the largest groups at risk for contracting hepatitis B virus (HBV) worldwide.
This is due to the accidental occupational exposure to potentially infectious blood and other body fluids in the workplace. This
cross-sectional study aimed to determine the rate of exposure to HBV infection and to identify potential occupational and nonoccupational
risk factors among doctors and nurses residing in tertiary hospitals in Sana’a city. This study included 169 physicians
and nurses of whom 121 were physicians and 48 were nurses. Blood samples were collected from each one, then tested for
serological markers of HBV infections. Also, data was collected in a pre-designed questionnaire including; demographic data, the
potential occupational and non-occupational risk factors that contribute to HBV transmission. The results of the study showed that
seropositive to hepatitis B surface antigen (HBsAg) among physicians and nurses was 5.3%, while the rate of exposure to hepatitis
B virus infection (HBcAb) was 17.8%. The rate of exposure to HBV infection (anti-HBC + HBsAg) was higher in females (33.3%)
than in males (21.4%).
The older age group was more susceptible to hepatitis B virus infection than the younger age group (P <0.05).Only 11
participants (6.5%) said they attended training courses in biosafety. Just over 45.6% indicated that they had needle injuries and
40% of sharp tool injuries while working; 61 (26%) indicated they always followed bio-safety precautions, and 74 (43.8%) said they
always wore gloves while their work. Only 32 (18.9%) of the participants received a full hepatitis B vaccination doses. Also, there
was a statistically significant relationship between cut injuries and HBV infections (P = 0.02). In addition, the highest incidence of
hepatitis B virus infection was 31.3% among nurses, while physicians had 19.8%. In conclusion, there was a high prevalence of
hepatitis B virus among doctors and nurses. Unfortunately, most workers have not received training in biosafety, and fewer than
half of the workers consistently use preventive measures such as wearing gloves during their work or taking vaccination. There is
a need to make health care workers vaccination against hepatitis B infection a consistent policy and to ensure full and consistent
compliance with standard safety procedures.
Keywords: HBV, resident, physicians, nurses, Yemen
Introduction
The hepatitis B virus (HBV) is the most dangerous type of viral hepatitis that causes a potentially life-threatening liver infection and leads to chronic liver disease and liver cancer [1]. HBV infection is a global public health problem and the tenth leading cause of death globally [2]. According to some estimates, nearly 2 billion people are infected with the hepatitis B virus worldwide, resulting in 400 million people worldwide infected with this chronic disease. Besides, more than a million deaths due to liver disease occur in the end stage, such as cirrhosis and liver cancer (HCC) every year [3]. Hepatitis virus endemicity was estimated to be high in Yemen, wherever positive HBsAg prevalence among adults was between 8% to 20%, among infants, 4.1%, and up to 50% of the population had serological evidence of hepatitis B virus infection in old reports [4-7]. On the other hand, recent studies have indicated a decrease in the rate of HBsAg as it ranges between 0.74-2% among the general population and blood donors as well as children [8-10]. When an occupational HBV was considered, the prevalence of hepatitis B virus among 388 public health center cleaners (PHCCs) was 8.2% [11].
HBV is carried in blood and other body fluids. Occupational exposure to blood and body fluids in hospitals leaves health care workers (HCWs) at risk of infection with blood-borne viruses including hepatitis B [8,12]. In 1992, the World Health Organization (WHO) recognized hepatitis B virus infection as an occupational disease for health sector workers [11]. Hospital residents, such as doctors and nurses, are at risk of infection with blood borne pathogens. This can be by a numerous procedure involving the use of sharp instruments on patients and injuries while learning new technical skill sets [11]. According to data provided by the World Health Organization, there are approximately 36 million health care workers worldwide, of whom about 3 million a year receive instrument injuries, and resulting to 2 million individuals infected with HBV, due to sharp injuries alone [13]. This cross-sectional study aimed to determine the rate of exposure to HBV infection and to identify potential occupational and non-occupational risk factors among doctors and nurses residing in tertiary hospitals in Sana’a city.
Subjects and Methods
This study included 169 randomly selected resident physician and nurses, of whom 121 were males and 48 were females and their age ranged from ≥22 to ≥38 years old with a mean age of 30 years. This study was conducted for a period of four months, starting in March 2018 and ending in June 2018 in Sana’a city. This study was performed at 3 tertiary hospitals in Sana’a city (Al-Jomhory, Al-Thorah, Al-Kuwait teaching hospitals). A consent form was done for each physicians and nurses in this study before withdrawing the blood specimens and the personal, occupational, and risk factors data were filled in a predesigned questionnaire About 4-5ml of venous blood was collected from each physicians and nurses in tubes containing separating gel and left to clot. Then all clotted samples were centrifuged at 3500 xg for 10 minutes. After that sera were divided in two labeled polypropylene screw – cap tubes and stored at -20 °C until tested for HBV markers. HBV markers were determined by using automatic sandwich electrochemiluminescence immunoassay (ECLIA) which intended for the use on the Elecsys 2010 analyzers machine, according to the manufacture information provided in the commercial kit manufactured by Rosh diagnostic Gmbh, Mannheim.
Statistical Analysis
Personal data and risk factors data were obtained from each subject and recorded in a pre-designed questionnaire, then the data were statistically analyzed by software version Epi Info version 6, CDC, Atlanta, USA. From two-by-two tables, the odds ratios were calculated and the value of P value was determined using the uncorrected chi square test. Fisher’s exact test was used for expected small cell sizes with a two-tailed probability value.
Results
Table 1 shows the demographic and occupational characteristics of the participants in the hepatitis B epidemiological survey, most of the individuals were physicians (121) and only 48 of the individuals tested were nurses. The number of males was 145 (85.8%) and 24 females (14.2%). Table 2 represents the prevalence and interpretation of serological markers of HBV, Susceptible HCWs in which they were negative for all markers counted 55%, immune after infection in which they are positive for anti-HB core and anti- HB surface antigen counted 17.8%, while immune after vaccination HCWs were 20.1% only in which anti-HBsAg were only positive. Current infections presented in 5.3% of total tested HCWs, while 1.8% was indeterminate. Table 3 shows the adjusted and odds ratio (risk) for contracting hepatitis B virus in various occupations, gender, and duration of work, when we considered positive against HBC + HBS-Ag (23.1%) as signs of contracting for hepatitis B virus, there was a high incidence of HBV infection among nurses (31.3%) with an OR value of 1.8, compared to 19.8% for physicians but this result was not statistically significant.
Table 1: Demographic and professional characteristics of the HBV survey participants, in tertiary hospitals, Sana’a city, Yemen.
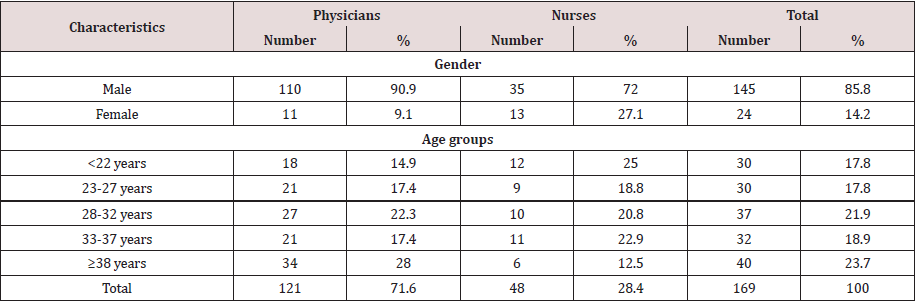
Table 2: Interpretation of serological markers of HBV among physicians and nurses in tertiary hospitals, Sana’a city, Yemen.
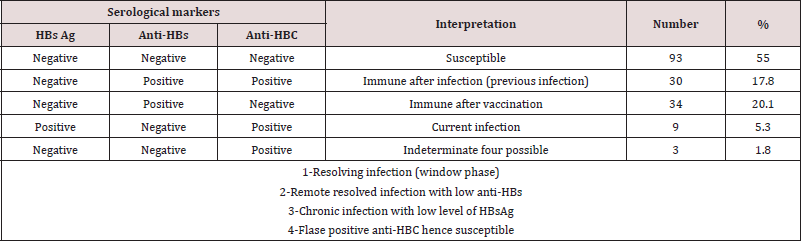
Table 3: The prevalent rate and odds ratio (risks) of contracting HBV for different occupations, gender, and duration of the work among physicians and nurses in tertiary hospitals, Sana’a city, Yemen.
χ2 Chi-square ≥ 3.84 (significant)
p Probability value < 0.05 (significant)
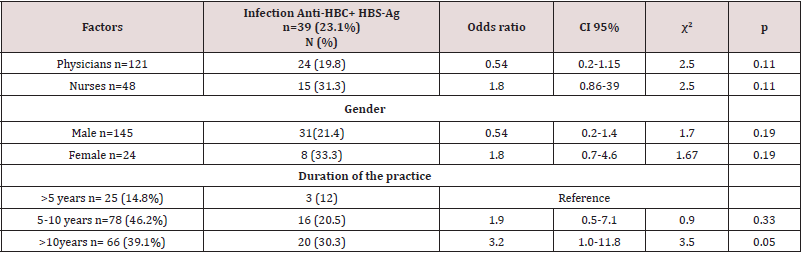
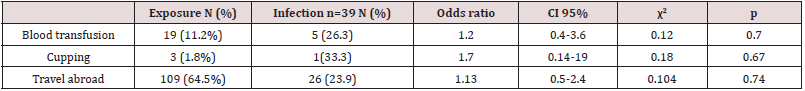
There was a higher rate of infection with female (33.3%) with an OR value of 1.8, compared to 21.4% for males but this outcome was not statistically significant. When we considered the duration of practice, there was a higher rate of contracting HBV for >10 years period (30.3%) with significant OR equal to 3.2 times, CI=1.0-11.8 times, comparing with >5 years period. Table 4 shows the occupational possible risk factors for HBV, There was a high rate of needle stick injuries (45.6%) and cuts (40.8%) among physicians and nurses. There was a higher rate of contracting HBV from occupational cuts (31.9%) with significant OR equal to 2.3 times, CI=1.2 – 4.7 times with X2=5.1 and P=0.02. There was a higher rate of contracting HBV from occupational needle stick injuries (27.3%) with non- significant OR equal to 2.5 times (P=0.23). Only 11 participants (6.5%) said they attended training courses in biosafety. Just over 45.6% indicated they had injuries and 40% of sharp tool injuries while working; 61 (26%) indicated that they always followed biosafety precautions, and 74 (43.8%) said they always wore gloves while working. Only 32 (18.9%) of the participants received a full hepatitis B vaccination dose. Table 5 shows the general risk factors for hepatitis B virus infection, and the prevalence of hepatitis B virus among individuals with a history of blood transfusion (26.3%), (OR = 1.2, p = 0.7). When cupping was considered as a risk factor, the prevalence of hepatitis B virus was 33.3%, with the risk association factor for hepatitis B contracting was equal to 1.7 and this result was not significant (p = 0.67). The prevalence rate among individuals with a history of traveling abroad was 23.9% with an OR = 1.13 (p = 0.74).
Table 4: Occupational possible risk factors for HBV among physicians and nurses in tertiary hospitals, Sana’a city, Yemen with previous and current HBV infection
χ2 Chi-square ≥ 3.84 (significant)
p Probability value < 0.05 (significant)
Table 5: General risk factors of contacting HBV among physicians and nurses in tertiary hospitals, Sana’a city, Yemen, with previous and current HBV infection.
χ2 Chi-square ≥ 3.84 (significant)
p Probability value < 0.05 (significant)
Discussion
The crude rate of HBs Ag that indicates the current infection
with the hepatitis B virus among our physicians and nurses is 5.3%
(Table 2). This rate is similar to the rate of the general population in
various regions in Yemen, including the city of Sana’a before 2004
[4-6]. However, our rate is five times higher than the rate that was
recently reported in the general population in different regions of
Yemen including adults and children, which ranges between 0.7-
2% among the general population [10,14,15]. This rate is similar
to the rate for dental clinics in the city of Sana’a, where the current
serological prevalence of hepatitis B virus infection was 6.1% [16].
The high rate of hepatitis B among HCWs in our study is similar
to the rate mentioned in previous epidemiological studies among
HCWs and is higher than the rate in the general population, and
this finding confirms that hepatitis B is an important occupational
hazard for health care workers [17,18]. In some studies, it has
been shown that HCWs have up to four times the risk of developing
hepatitis B virus [19]. As the main risk factor for infection with
hepatitis B virus for HCWs is direct contact with infectious
substances, especially blood infected with HBV or via a needle
stick injury with body fluids contaminated with hepatitis B virus
as described by Abbas et al. [16] In Yemen and Pellissier et al. [20]
In Nigeria.
In particular, recapping of hollow-bore needles appears to
increase the risk of needle stick injuries [21]. Other studies have
reported a lack of awareness of HBV among HCWs; consequently,
proper precautions (e.g., use of disposable gloves) against bloodborne
infections are lacking in these workers [1]. This observation
is consistent with other studies demonstrating that untrained
individuals are more likely to be exposed to HBV infection [22].
The prevalence of current HBV infection among female health care
workers in our study was 7.8%; prevalence of life time exposure
to hepatitis B virus was 33.3%; higher than that of males as the
current hepatitis B infection was 4.2%; the prevalence of a lifetime
exposure was 21.4%. Also the associated odds ratio HBV infection
in females was 1.8 times, compared to 0.54 for males (Table 3).
This result differs from the common pattern of HBV among HCWs
in most reports where the rate of HBV is almost the same in both
sexes, [17.23] while this result is similar to that reported by Abbas
et al. [16] in Yemen, where the infection rate is higher among female
HCW than male HCWs. This result might be explained by that female HCWs exposed more than male HCWs to the risk factors of
contracting HBV [23,18].
The results of our study indicate that the prevalence of HBV
depends on the period of practice in the profession, where rates
increase with increasing duration of practice, for example the
rate of antibodies to HBs + HBs Ag for> 5 years was 12% and
this percentage increased for a period of 10 years to 33.3%, with
significant associated OR equal to 3.2 times (pv=0.05) (Table 3). This
results is similar to studies that covered wider range of duration of
practices in several risk HCW groups including physicians, nurses
and dentists which indicated that the prevalence of HBV is duration
practice dependent, in which it was increased with increasing
duration of practice HCWs occupation age [16,17,21,23]. This
relation could be explained by various reasons. One explanation
could be that there is a more or less constant risk of exposure during
life time and therefore the Hepatitis B prevalence increases with
time of exposure. We cannot rule out, that the risk of transmission
might have changed over time due to increased awareness and
precautions like wearing of gloves and use of safety needles. On
the other hand the finding, that long occupational exposure in
healthcare services increases the risk of acquiring HBV infection, is
consistent with other studies [17,23].
The risk of acquiring HBV from a needle stick injury ranges from
1% to 6% (source patient HBsAg-positive, HBeAg-negative) to 22%
to 40% (source patient HBsAg-positive, HBeAg-positive) [24]. The
risk of non-percutaneous exposure may account for a significant
proportion of HBV transmission in the healthcare setting. Hepatitis
B virus can survive in dried blood for up to a week and thus may be
transmitted via discarded needles or fomites, even days after initial
contamination. Indeed, many healthcare workers infected with
HBV cannot recall an overt needle stick injury, but can remember
caring for a patient with hepatitis B [25]. There was a significant
risk of infection with hepatitis B virus in our HCWs with history of
recent accident cut during practices where the OR was 2.3 times
and this outcome was important where p = 0.02 (Table 4). This
finding shows that our medical professionals may be more likely
to get hepatitis B infection in hospitals because they are learning to
do procedures and may be less cautious than other health workers
in other countries. They are also less likely to practice universal
precautions and are more likely to sustain needle stick injuries due
to inexperience.
The present study showed that Medical workers (physicians
and nurses) of face a high risk of blood-borne infections through
blood exposures incidents. The prevalence rate needle stick was
45.6%. In a study conducted in Australia, an average of 3.0 percutaneous
exposures (PCE) was reported among physicians
annually [24]. Difference in exposure rates among different
studies may be due to different subjects (job categories), sampling methodologies. Medical workers in Yemen represent the most staff
and less experienced, and hence longer working hours and greater
probability of blood exposure. A study was conducted among
Australian medical workers in whom 13.8% had suffered a total of
41 needle stick and sharps injuries (NSI) incidents [24]. In 2003 a
study was conducted in Missouri, USA, in which 43 out of 224 HCWs
(19.2 %) reported needle stick injuries [26]. Needle stick injuries
during internship were reported by 61.9% (438/708) of Taiwanese
nurses [27]. In the above-mentioned studies, it seems that in the
more developed countries, the number of blood-exposure accidents
lends to be lower. The overall socio-economic status and knowledge,
and adoption of necessary precautions, and safety guidelines have
led to lower exposure rates.
Presently in Yemen, efforts aimed at controlling hepatitis
B viral infection remain feeble. There are no policies at both the
National and Institutional levels on vaccination of high risk groups
like health care workers and medical students. The present study
was carried out also to determine the hepatitis B vaccination
rate among medical workers in hospitals who could readily come
in contact with infected body fluids from patients and hospital
equipment during their clinical workers. This will generate
information required to advocate for pre-vaccination policies for
all high risk groups. Also, immunization against hepatitis B viral
infection has assumed a primary role in the control of hepatitis B
infection. Hepatitis B vaccine has been found to effectively reduce
the prevalence of HBV infection [8,10]. Several studies [8,9,18]
demonstrated that introduction of compulsory HBV vaccination
contributes in decreasing HBV incidence rates. After a standard
3-dose vaccination regime at 0, 1, and 6 months, the rate of
response on the basis of an anti-HBsAg titer of ≥10 mIU/mL is
90%–95% [18,28,29]. Unfortunately, a significant proportion of
health care workers including physicians and nurses do not receive
HBV immunization, and remain susceptible to HBV infection [28].
Vaccination coverage of the medical workers in the present work
was 49.1% (one or more doses) against HBV and only 20.1% of the
total were immune after vaccination (Table 4). Among Taiwanese
nurses, vaccination against hepatitis B virus (HBV) was lacking
in 47.6% [27]. However, the effectiveness of the vaccination is
an important factor; also the completed doses should be strictly
followed. In our study we found that the lowest vaccination rate
(25%) (Table 4) was among the nurses while vaccination rate
among physicians was higher (58.7%).
Also the study findings showed that 6.2% of all vaccinated
individuals had full vaccine doses were regarded as infected with
HBV infection (HBsAg+Anti-HBC positive) (Table 4). Different
findings were reported in Iran among vaccinated adults, where a
high protective anti-HBs response rate was found among vaccinated
adults (97.4%) [29]. This difference in findings could be attributed
to a different response in the primary course of vaccination, different age groups, or to the different degrees of exposure to natural
boosters and nutritional status and socioeconomic factors, race
factors, or the type of vaccines used [30]. In this study, HB surface
antigen was obtained among the whole studied HCWs (vaccinated
and non-vaccinated), but due to the lack of serological data, either
before or after vaccination, it was impossible to conclude whether
these HCWs were already infected at the time of vaccination or
infected subsequently. In the present study it was found that the
frequency of HBsAg+anti-HBc positivity among the whole HCWs
were 23.1% (Table 2), which was lower among full dose vaccinated
(6.2%) when compared with the rate of the non-vaccinated HCWs
(32.6%) (Table 4). This result indicate absent of HBV vaccine is
risk factor for contracting HBV infection and vaccination for HBV
is protective measures against HBV infection as described by most
previous reports [9,10,18,31].
Also one of our aim was to determine the non-occupational risk
factors of contracting hepatitis B virus among our HCWs. To achieve
this aim, odds ratio of contracting HBV infection, and its confidence
interval was calculated, and their significant also was determined
by X2 and p value (Table 5). There was no significant association
between HBV contract with history of blood transfusion, cupping
and/or travel abroad, and this different with findings among
different population groups in Yemen by Al-Shamahy et al. [4],
and Scot et al. [6] that prior factors were significant risk factor for
hepatitis virus infections. Our results were also different from those
conducted in Syria, where the previous factors were the risk factors
for hepatitis B virus infection among the general population and
risk groups in Syria [32].
Conclusion
In conclusion high prevalence rates of HBV occurred among physicians and nurses. Unfortunately; most of the workers did not take training on biosafty, and less than half of the workers use protective measures consistently as always wore gloves during their work or vaccination. There is needed to make vaccination of health care workers against HBV infection a firm policy and ensure complete and consistent adherence to work standard safety measures. Also further research is needed to clarify the results of the current study.
Author’s Contribution
This research work is part of a research work under the supervision of Hassan Al-Shamahy. The field, and laboratory works of the research was done by the corresponding author, and the forth author. The first, second, and third authors supervised the work and edited the manuscript.
Acknowledgments
The authors thank the University of Sana’a for financial support
Conflict of Interest
“There is no conflict of interest related to this work.”
References
- Ott JJ, Stevens GA, Groeger J, Wiersma ST (2012) Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 30: 2212.
- Yousafzai MT, Qasim R, Khalil R (2014) Hepatitis B vaccination primary health care workers in Northwest Pakistan. International Journal of Health Sciences 8(1): 68-76.
- Khedive A, Sanei E, Alavian SM (2013) Hepatitis B virus surface antigen (HBsAg) mutations are rare but clustered in immune epitopes in chronic carriers from Sistan-Balouchestan Province. Iran. Arch Iran Med 16(7): 385-389.
- AL-Shamahy H (2000) Prevalence of Hepatitis B surface antigen and Risk factors of HBV infection in samples of healthy mothers and their infants in Sana'a, Yemen. Ann Saudi Medicine 20: 464-467.
- Al-Shamahy HA, IA Rabbad, A Al-Hababy (2003) Hepatitis B virus serum markers among pregnant women in Sana'a, Yemen. Ann Saudi Med 23: 87-89.
- Scott DA (1990) A sero epidemiological survey of viral hepatitis in Yemen Arab Republic. Transactions of the Royal Society of Tropical Medicine and Hygiene 84(2): 288-291.
- Al-Waleedi AA, Khader YS (2012) Prevalence of hepatitis B and C infections and associated factors among blood donors in Aden city, Yemen. Eastern Med Health J 18(6): 1-7.
- Al-Shamahy HA, Jaadan BM, Al-Madhaji AG, Al-Fraji BBM, Ajrah MA, et al. (2019) Prevalence and potential risk factors of hepatitis B virus in a sample of children in two selected areas in Yemen. Universal Journal of Pharmaceutical Research 4(3): 18-22.
- Al-kadassy AM, Al-Shamahy HA, Al-Ashiry AFS (2019) Sero-epidemiological study of hepatitis B, C, HIV and treponema pallidum among blood donors in Hodeida city- Yemen. Universal Journal of Pharmaceutical Research 4(2): 40-44.
- Al-Shamahy HA, Samira H Hanash, Iqbal A Rabbad, Nameem M Al-Madhaji (2011) Hepatitis B Vaccine Coverage and the Immune Response in children under 10 years old in Sana'a Yemen. SQU Med J 11(1): 77-82.
- AL-Marrani WHM, Al-Shamahy HA (2018) “Prevalence of HBV and HCV; and their associated risk factors among public health center cleaners at selected public health centers in Sana’a city-Yemen”. Universal Journal of Pharmaceutical Research 3(5):1-5.
- Franco E, Bagnato B, Marino MG (2012) Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol 4(3): 74-80.
- Elseviers MM, Arias-Guillén M, Gorke A (2014) Sharps injuries amongst healthcare workers: review of incidence, transmissions and costs. J Ren Care 40: 150-156.
- Murad EA, Babiker SM, Gasim GI, Rayis DI, Adam I (2013) Epidemiology of hepatitis B and hepatitis C virus infections in pregnant women in Sana’a, Yemen. BMC Pregnancy and Childbirth 13: 113-127.
- Nabehi BAH, Al- Shamahy H, Saeed WSE, Musa AM, El Hassan AM, et al. (2018) Sero-molecular epidemiology and risk factors of viral hepatitis in urban Yemen. Int J Virol 11: 133-138.
- Abbas Al Kasem MA, Al-Kebsi Abbas M, Madar Ebtihal M, Al-Shamahy Hassan A (2018) Hepatitis B virus among dental clinic workers and the risk factors contributing for its infection. On J Dent Oral Health 1(2): 1-6.
- Jha AK, Chadha S, Bhalla P, Saini S (2012) Hepatitis B infection in microbiology laboratory workers: prevalence, vaccination, and immunity status. Hepat Res Treat pp. 520362.
- WHO (2016) Health Care Worker Safety/AIDE-Memoire for a strategy to protect health workers from infection with blood borne viruses.
- Schweitzer A, Horn J, Mikolajczyk RT (2015) Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 386: 1546.
- Pellissier G, Yazdanpanah Y, Adehossi E, Tosini W, Madougou B, et al. (2012) Is universal HBV vaccination of healthcare workers a relevant strategy in developing endemic countries? The case of a university hospital in Niger. Plosone 7: e44442.
- Salehi AS, Garner P (2010) Occupational injury history and universal precautions awareness: a survey in Kabul hospital staff. BMC Infect Dis 10: 19.
- Ghany MG, Perrillo R, Li R (2015) Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol 13: 183-192.
- Noubiap JJN, Nansseu JRN, Kengne KK, Wonkam A, Wiysonge CS (2014) Low hepatitis B vaccine uptake among surgical residents in Cameroon. Int Arch Med 7: 11.
- Smith DR, Leggat PA (2005) Needlestick and shaips injuries among Australian medical students. J UOEH 27: 237-242.
- Sepkowitz KA (1996) Occupationally acquired infections in health care workers. Part II. Ann. Intern. Med 125: 917-928.
- Patterson JM, Novak CB, Mackinnon SE, Ellis RA (2003) Needle stick injuries among medical students. Am J Infect Control 31(4): 226-230.
- Shiao Judith SC (2002) Student Nurses in Taiwan at High Risk for Needle stick Injuries. Annals of Epidemiology 12(3):197-201.
- Zanetti AR, Van Damme P, Shouval D (2008) The global impact of vaccination against hepatitis B: a historical overview. Vaccine 26(49): 6266-6273.
- Guo Y, Hui CY (2009) Pilot-scale production and quality control of multiepitope hepatitis B virus DNA vaccine. Nan Fang Yi Ke Da Xue Xue Bao 29(1): 118-120.
- Greengold B, Nyamathi A, Kominski G (2009) Cost-effectiveness analysis of behavioral interventions to improve vaccination compliance in homeless adults. Vaccine 27(5): 718-725.
- Jung M, Kuniholm MH, Ho GY (2016) The distribution of hepatitis B virus exposure and infection in a population-based sample of U.S. Hispanic adults. Hepatology 63: 445-452.
- Moukeh G, Yacoub R, Fahdi F (2009) Epidemiology of hemodialysis patients in Aleppo city. Saudi J Kidney Dis Transpl 20(1): 140-146.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




