
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2643-6760
Research Article(ISSN: 2643-6760) 
Coexistence of A Secondary STRN-ALK EML4-ALK Double- Fusion Variant in A Lung Adenocarcinoma Patient with EGFR Mutation: A Case Report Volume 6 - Issue 1
Qian Zeng MD1, Han Gao MD2, Longan Zhang MD1, Shouming Qin BD2, Yongyao Gu MD3 and Quan Fang Chen MD2*
- 1Department of Emergency, The First Affiliated Hospital, Guangxi Medical University, PR China
- 2Institute of respiratory disease, The First Affiliated Hospital, Guangxi Medical University, PR China
- 3Department of Pathology, The First Affiliated Hospital, Guangxi Medical University, PR China
Received: January 07, 2021; Published: January 29, 2021
Corresponding author: Quan Fang Chen, Institute of Respiratory Disease, The First Affiliated Hospital Guangxi Medical University, No.6 Shuangyong Road, People’s Republic of China
DOI: 10.32474/SCSOAJ.2021.06.000229
Abstract
Anaplastic lymphoma kinase (ALK)-positive disease is characterized by the presence of ALK gene rearrangements that encode driver fusion oncoproteins. Echinoderm microtubule-associated protein-like 4 gene (EML4)-ALK fusion is regarded as the most common type and is reported in 2 to 7% of patients with advanced non–small cell lung cancers (NSCLCs). Striatin (STRN)-ALK is a novel ALK fusion partner in NSCLC and is considered sensitive to targeted therapy. However, there was no study regarding effective therapy for EML4-ALK and STRN-ALK double fusion variants in epidermal growth factor receptor (EGFR)-resistant mutant lung cancer. TP53, RB1, and EGFR exon 21 L858R were found in tumor tissues and plasma from patients with capture-based nextgeneration sequencing (NGS). After three months of gefitinib treatment, an NGS of plasma circulating tumor DNA (CTDNA) showed that all variants disappeared significantly, and the tumor mass regressed on computed tomography (CT). However, after 10 months, the patient developed drug resistance and the disease progressed with the appearance of new metastatic lesions in the liver and bones. A repeated NGS test revealed EGFR exon20 T790M and the appearance of a novel double-fusion EML4-ALK and STRNALK. A combined therapeutic regimen of crizotinib plus osimertinib showed a promising prognosis confirmed with lung CT scans showing stable lesion without any new metastasis. Moreover, a subsequent genotype by NGS also showed the disappearance of STRN-ALK and EGFR exon20 T790M. The therapeutic efficacy of crizotinib plus osimertinib on EML4-ALK and STRN-ALK doublefusion variant in patients with EGFR resistant mutant lung cancer may provide a supportive reference for the patients with such genetic alteration. NGS might contribute to optimizing the selection of patients.
Keywords: STRN-ALK; EML4-ALK; double-fusion; gefitinib; crizotinib; osimertinib
Abbreviations: ALK: Anaplastic Lymphoma Kinase; EML4: Echinoderm Microtubule-Associated Protein-like 4 gene; NSCLC: Non–Small Cell Lung Cancer; STRN: Striatin; EGFR: Epidermal Growth Factor Receptor; NGS: Next-Generation Sequencing; CTDNA: Circulating Tumor DNA CT: Computed Tomography; TKI: Tyrosine Kinase Inhibitors
Introduction
Studies reporting the coexistence of EGFR and anaplastic lymphoma kinase genes (ALK) in a single patient has challenged the previously established theory, which states that EGFR mutation is mutually exclusive to ALK rearrangement. However, such findings reported with double ALK fusion simultaneously in one patient with EGFR mutation is still rare. Herein, we presented a secondary striatin (STRN)-ALK, echinoderm microtubule-associated protein-like 4 gene (EML4)-ALK double-fusion variant in lung adenocarcinoma with EGFR mutations that responded to gefitinib.
Case Presentation
A 38-year-old male nonsmoker presented to the hospital with chest pain, dyspnea, and lumbago. A chest computed tomography (CT) scan showed a mass in the left lung, multiple nodules in the right lung with pleural effusion in small quantities (Figure 1A). The brain CT scan was normal. Bone radionuclide CT scan showed multiple bone metastases. Bronchoscopy biopsy revealed atypical cells in the submucosa of the left bronchus. Immunohistochemistry showed positivity for thyroid transcription factor 1, napsin A, and negativity for P40. A capture-based next-generation sequencing (NGS), as well as plasma circulating tumor DNA (ctDNA) detection, were recommended from the bronchoscopy biopsy samples of tumor tissue. TP53, RB1, EGFR exon21L858R were identified in tissue (49.6% abundance, 40.4% abundance and 78.0% abundance) and plasma ctDNA (10.2% abundance, 9.3% abundance and 28.4% abundance) (Table 1). On March 25, 2019, the treatment with gefitinib was initiated (250 mg twice daily). After 3 months of gefitinib therapy, a follow-up CT scan revealed that partial response was achieved with evidence of a significant reduction in tumor size and shrinkage of nodules in the right lung (Figure 1A). Meanwhile, NGS of plasma ctDNA showed the disappearance of all variants (Figure 1B). After 6 months, the disease was evaluated as stabilized and the administration of gefitinib was continued. On April 21, 2020, a CT scan showed that the lung lesion was still stable but with liver metastases and a new appearance of bone metastases. The disease was evaluated as a progressive disease. Simultaneously, NGS assay of patient’s plasma ctDNA identified a double ALK fusion: STRN-ALK (S3:A20, 0.70% abundance), EML4-ALK (E2:A20, 0.90% abundance) and new EGFR (exon 20 T790M, 0.80% abundance) in addition to the recurrence of the original TP53, RB1 and EGFR exon 21del (7.0% abundance, 9.4% abundance and 25.9% abundance) (Table 1 and Figure 1C). Based on the above clinical parameters, we prescribed combination therapy with crizotinib and osimertinib. Further lung CT-scan evaluation in June and September 2020 showed that the lesion was confined to its original location without any signs of new metastasis in other areas including liver and bone. (Figure 1A). A repeated NGS assay in September detected TP53, RB1, EGFR exon21, EML4-ALK (22% abundance, 24.8% abundance, 46.2% abundance, and 14% abundance), while the STRN-ALK and EGFR T790M variants were not detected.
Figure 1: (A) CT images at different phases of treatment. (B) Mutation abundance using the next-generation sequencing (NGS) of circulating tumor DNA (ctDNA). 1, NGS of the patient’s plasma ctDNA for the first time on March 25, 2019; 2, NGS of the patient’s plasma ctDNA for the second time on June 19, 2019; 3, NGS of the patient’s plasma ctDNA for the third time on April 21, 2020; 4, NGS of the patient’s plasma ctDNA for the fourth time on September 18, 2020. (C) The fusion pattern of echinoderm microtubule associated protein like 4 gene (EML4)-ALK receptor tyrosine kinase (ALK) and striatin (STRN)–ALK.
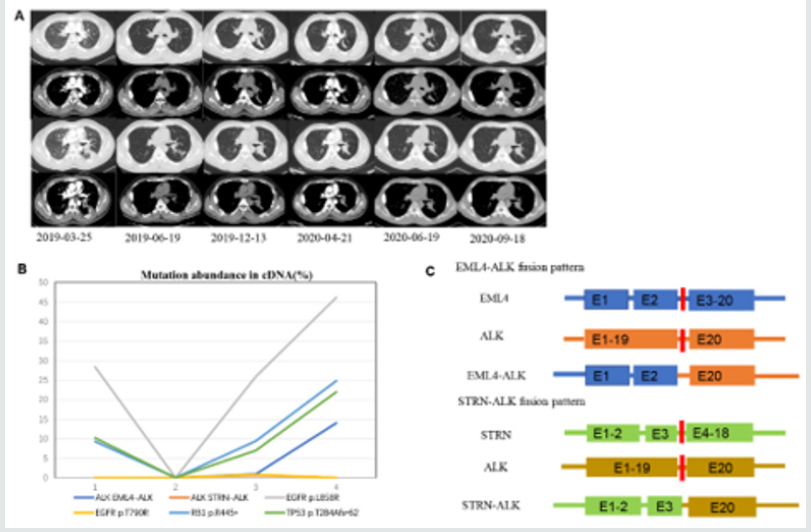
Table 1: Changes in liquid biopsy.

ND: Not Detected; c.HGVS: Description of Coding DNA (c.) HGVS: variants by Human Genome Variation Society; p.HGVS, description of protein (p.) variants by HGVS. *describe a stop codon.
Discussion
The most common fusion partner in ALK-rearranged NSCLC is EML4 [1], while the STRN-ALK fusion occurs very rarely. Up till now, only five cases of STRN-ALK fusion in lung cancer have been reported. Functionally, Variant 5 (exon 2 of EML4 was connected to exon 20 of ALK) which is peculiar with its coiled-domain play a critical role in the dimerization and activation of EML4- ALK subtypes and in binding EML4-ALK to certain subcellular components [2]. Proportionately, the fusion product of EML4 with ALK kinase domains varies with the extent of fusion [3]. To the best of our knowledge, this is the first study to report the STRN-ALK, EML4-ALK double fusion, and the coexistence of double fusion and EGFR mutation in a lung adenocarcinoma patient. Secondary resistance of EGFR exon20 T790M is most commonly found in firstline EGFR-tyrosine kinase inhibitors (TKI), but it responds well to osimertinib [4]. STRN and ALK are situated on the same short arm of chromosome 2 as EML4 [5]. Recently, STRN-ALK fusion protein has been identified as a potential therapeutic target in many cancer types, including those of few highly aggressive cancers of the thyroid, colorectal and renal cell carcinomas, in addition to cancers of the liver and lungs. Moreover, these carcinomas show similar invasive features with extra-organ and lymph node metastasis [6- 8]. It was reported that the thyroid and colorectal cancer patients responded significantly well to crizotinib. STRN-ALK–transfected rat thyroid cells were sensitive to crizotinib based on an in vitro experiment, but the sensitivity to alectinib or other ALK inhibitors was unclear [9]. It was reported that an NSCLC patient harboring STRN-ALK fusion without ALK-resistant mutation showed resistance to alectinib therapy and died 6 months after the initial diagnosis [5]. It was regarded that STRN-ALK fusion was sensitive to crozotinib with exceptionally long survival but was unresponsive to alectinib [4]. However, there is no data on the sensitivity of EML4- ALK and STRN-ALK mutations in EGFR-resistant mutant NSCLCs to the combination therapy of crizotinib and osimertinib. Recently, the use of crizotinib to inhibit ALK has become the standard therapeutic strategy in advanced ALK-rearrangement NSCLC, but ALK fusion forms vary widely in their curative effect and duration of response. Even with the specific EML4-ALK fusion variant, it will show different sensitivity to crizotinib in vitro [1]. Although the exact function remains unclear, EMLs are considered to represent a type of inhibitor modulating the microtubule function in association with its ability to restrain the cellular proliferation and mitosis [3].
Conclusion
As for the existing patient, we hypothesized that STRN-ALK fusion was the main cause of extrapulmonary metastasis in lung cancer. We speculated that gefitinib can be an ideal alternative therapeutic target to supplant Osimertinib in patients with the nonexistence of EGFR exon 20 T790M reported with drug resistance. Besides, our case study also demonstrated that plasma CTDNA analysis can be effectively applied to detect the variant type and to further predict and complement the efficacy of TKI in NSCLC based on the link between variant and clinical outcome. Collectively, our data may provide supporting evidence and guidance for implementing an effective therapeutic strategy for similar cases.
References
- Woo CG, Seo S, Kim SW, Jang SJ, Park KS et al. (2017) Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Annals of oncology official journal of the European Society for Medical Oncology 28: 791-797.
- Takeuchi K, Choi YL, Soda M, Inamura K, Togashi Y, et al. (2008) Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clinical cancer researches an official journal of the American Association for Cancer Research 14(20): 6618-6624.
- Früh M, Peters S (2018) EML4-ALK Variant Affects ALK Resistance Mutations. Journal of clinical oncology official journal of the American Society of Clinical Oncology 36(12): 1257-1259.
- Zhou C, Zeng L, Zhang Y, Yang N (2019) Responder of Gefitinib Plus Crizotinib in Osimertinib Failure EGFR-mutant NSCLC-Resistant with Newly Identified STRN-ALK by Next-Generation Sequencing. Journal of thoracic oncology official publication of the International Association for the Study of Lung Cancer 14(7): e143-e144.
- Nakanishi Y, Masuda S, Iida Y, Takahashi N, Hashimoto S (2017) Case Report of Non-Small Cell Lung Cancer with STRN-ALK Translocation: A Nonresponder to Alectinib. Journal of thoracic oncology official publication of the International Association for the Study of Lung Cancer 12(12): e202-e204.
- Kelly LM, Barila G, Liu P, Evdokimova VN, Trivedi S, et al. (2014) Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proceedings of the National Academy of Sciences of the United States of America 111(11): 4233-4238.
- Yakirevich E, Resnick MB, Mangray S, Wheeler M, Jackson CL, et al. (2016) Oncogenic ALK Fusion in Rare and Aggressive Subtype of Colorectal Adenocarcinoma as a Potential Therapeutic Target. Clinical cancer researches an official journal of the American Association for Cancer Research 22(15): 3831-3840.
- Majewski IJ, Mittempergher L, Davidson NM, Bosma A, Willems SM, et al. (2013) Identification of recurrent FGFR3 fusion genes in lung cancer through kinome-centred RNA sequencing. The Journal of pathology 230(3): 270-276.
- Ren H, Hou X, Eiken PW, Zhang J, Pierson KE, et al. (2019) Identification and Development of a Lung Adenocarcinoma PDX Model With STRN-ALK Fusion. Clinical lung cancer 20(2): e142-e147.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




