Lupine Publishers Group
Lupine Publishers
Research Article(ISSN: 2644-1306) 
Clinical Significance of HIF-1α and Hs-CRP in COPD Disease Progression Volume 1 - Issue 5
Lijun Chen1, Juanxia Chen1, Huifang Zhang1, Mei fang Liu1, Liting Ma1, Chunyan Xia1 and Xiaoyong Ma2*
- 1Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Ningxia Medical University, The First People’s Hospital of Yinchuan, Yinchuan, Ningxia, China
- 2Department of Traditional Chinese Medicine, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
Received:January 30, 2022; Published: February 16, 2023
*Corresponding author:MA Xiaoyong, Department of Traditional Chinese Medicine, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
DOI: 10.32474/TRSD.2023.01.000121
Abstract
Objective:a) To analyze the correlation between serum Hypoxia-inducible Factor (HIF)-1α, Hypersensitive C-reactive Protein (Hs-CRP),
and lung function in patients with Chronic Obstructive Pulmonary Disease (COPD).
b) To investigate the clinical significance of HIF-1α and Hs-CRP on COPD disease progression. Methods One hundred and sixty
COPD patients attending the Department of Respiratory and Critical Care Medicine of the First People’s Hospital of Yinchuan
from 2016 to 2020 were selected and divided into four groups of mild, moderate, severe, and very severe based on pulmonary
function tests, with 40 cases in each group. The HIF-1α and Hs-CRP concentrations and pulmonary function of all enrolled
patients were measured.
c) The differences in HIF-1α and Hs-CRP and the correlation between HIF-1α, Hs-CRP, and FEV1% among the four groups were
analyzed.
Results:a) HIF-1α and Hs-CRP concentrations gradually increased with increasing disease severity in COPD patients, with statistically
significant differences (p<0.001).
b) HIF-1α was positively correlated with Hs-CRP and negatively correlated with FEV1% in all four groups of patients (p<0.05),
and the correlation coefficients increased with increasing disease severity.
Conclusion:Hypoxia and inflammation interact and contribute to disease progression in COPD patients.
Keywords:HIF-1α; Hs-CRP; COPD; Disease Progression; Hypoxia; Inflammation
Abbreviations:COPD: Chronic Obstructive Pulmonary Disease; HIF: Hypoxia-inducible Factor.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) leads to airway remodeling and airflow limitation due to chronic inflammation of both lungs and bronchi. With the progression of the disease, lung parenchyma and pulmonary blood vessels are further damaged, leading to ventilation/blood flow imbalance and hypoxemia. Under hypoxic stimulation, the overexpression of HIF-1α regulates certain inflammatory factors to participate in the inflammatory disorder of COPD [1-3], which causes inflammation to persist or amplify. At the same time, inflammatory factors can activate the expression of HIF-1α [4], resulting in a vicious cycle of “inflammation, hypoxia and inflammation” and thus promoting the progression of COPD. In this study, the serum levels of HIF-1α and Hs-CRP and lung function in patients with different severity of COPD were detected, and the differences of HIF-1α and Hs-CRP between patients with different severity of COPD and the correlation between HIF-1α and Hs-CRP, FEV1% were analyzed. To investigate the possible role of HIF-1α and Hs-CRP in the progression of COPD.
Materials and Methods
General Information: A total of 160 patients with COPD who were treated in the Department of Respiratory and Critical Care Medicine of the First People’s Hospital of Yinchuan from 2016 to 2020 were collected. According to pulmonary function test, they were divided into mild, moderate, severe, and very severe groups, with 40 cases in each group. All patients provided written informed consent.
Inclusion Criteria: Eligible patients met the diagnostic criteria of COPD in the 2020 GOLD guidelines, and patients with bronchial asthma, active tuberculosis, lung cancer, primary bronchiectasis, pneumoconiosis, and other pulmonary restrictive ventilation dysfunction were excluded.
Pulmonary function grouping criteria: Mild (grade I): FEV1/ FVC < 70%, FEV1% predicted≥ 80%; Moderate (grade Ⅱ): FEV1/ FVC<70%, 50% ≤FEV1% predicted< 80%; Severe (grade Ⅲ): FEV1/ FVC < 70%, 30%≤FEV1%predicted < 50%; Very severe (grade IV) : FEV1/FVC< 70%, FEV1 % predicted< 30%, or with chronic respiratory failure.
Collection of specimens: On the day of enrollment, 5ml peripheral venous blood was drawn from all patients, anticoagulated with EDTA, and the upper serum was extracted after centrifugation and stored in the refrigerator at -80℃ for further use.HIF-1α detection: Human HIF-1α enzyme-linked immunosorbent assay kit was used to detect serum HIF-1α.
Hs-CRP detection: Serum Hs-CRP concentration was measured by turbidimetry.
Pulmonary function tests: HP Master Scope pulmonary function instrument was used to test the pulmonary function of the patients.
Statistical methods: SPSS22.0 For Windows software package was used to analyze the research data. The continuous variable data of each group were expressed. As mean ± standard deviation (X ± s). One-way analysis of variance was used for comparison between groups, q test was used for comparison between groups, and Chisquare test was used for counting data. P < 0.05 was statistically significant.
Results
The general data and concentrations of HIF-1 α and Hs- CRP were compared among the four groups.
There were no significant differences in age, height, and gender among the four groups, P﹥0.05, which is comparable. With the increase of disease severity, the concentrations of HIF-1αand Hs-CRP in the four groups gradually increased, and the statistical analysis showed that the differences were statistically significant (p < 0.001) Table 1.
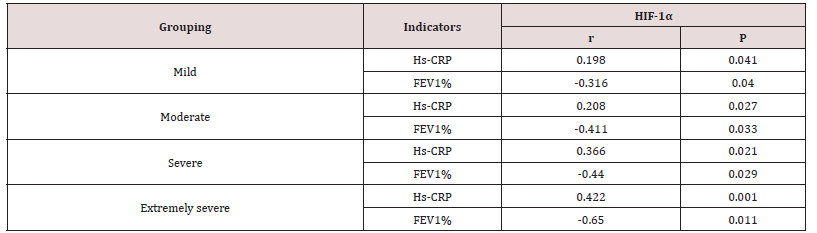
Correlation analysis of HIF-1α, Hs-CRP and FEV1% in the four groups
The linear correlation analysis of HIF-1α with Hs-CRP and FEV1% showed that HIF-1α was positively correlated with Hs- CRP and negatively correlated with FEV1% in the mild/moderate/ severe/extremely severe groups, and the correlation increased with the aggravation of the disease Table 2.
Discussion
COPD is a chronic respiratory disease involving airway, pulmonary parenchyma, and pulmonary blood vessels. Its core is systemic inflammatory response, and inflammation plays a key role in the occurrence and development of COPD [5,6].The process of airway wall injury and repair occurs repeatedly under the stimulation of chronic inflammation, which leads to airway remodeling, increased collagen content of lung parenchyma and scar formation. The above pathological changes eventually lead to irreversible airflow limitation of the airway, imbalance of ventilation/blood flow in the lung, and tissue hypoxia. Under the stimulation of inflammation and hypoxia, the body leads to vascular endothelial injury, erythropoietin overexpression, blood viscosity, thrombosis, and gradually appear pulmonary heart disease, osteoporosis, gastroesophageal reflux, sarcopenia and other multi-system complications, which accelerate the progress of COPD disease. HIF-1α is a sensitive indicator of hypoxia, which is rapidly expressed under hypoxia and rapidly degraded under normoxia. Under hypoxia, HIF-1α regulates the body to adapt to the hypoxic environment by regulating the expression of a series of hypoxia genes, thereby avoiding tissue and cell damage caused by hypoxia [7-9].In order to adapt to long-term hypoxia, COPD patients gradually develop complications such as hypoxic pulmonary hypertension and pulmonary heart disease[10].
This study found that the expression of HIF-1α was different in the four groups of COPD patients with different disease severity, and the expression of HIF-1α increased gradually with the increase of disease severity, and the difference was statistically significant, suggesting that the increase of disease severity was accompanied by the increase of the degree of hypoxia. Therefore, clinical monitoring of HIF-1α concentration can more accurately assess the body’s hypoxia status, indirectly reflect the degree of disease progression, and provide objective basis for clinical oxygen therapy [2]. Hs-CRP is a biomarker with high sensitivity and good predictive value for inflammation, and it is easy to obtain in clinical practice, even in primary medical and health institutions, it is also easy to carry out to guide clinical diagnosis and treatment’s-CRP can induce a series of inflammatory cytokines by activating NF-B signaling pathway [11,12], thereby aggravating the inflammatory effect of COPD and promoting disease progression. Many studies have confirmed that Hs-CRP plays an important role in assessing disease severity, acute exacerbation risk and prognosis of COPD patients [13-15].This study showed that the concentration of Hs-CRP in COPD patients was in the very severe group > severe group > moderate group > mild group, indicating that with the progression of the disease, the inflammatory response of COPD was gradually aggravated. We conducted a linear correlation analysis between HIF-1α and Hs- CRP and FEV1% in the four groups of COPD patients.
The results showed that HIF-1α was positively correlated with Hs-CRP and negatively correlated with FEV1% in the four groups of patients, and the correlation increased with the severity of the disease. This result implies that COPD hypoxia and inflammation are inseparable. Relevant studies have also confirmed that inflammatory factors such as TNF-α and IL-6 activate HIF-1α under hypoxia, and HIF-1α aggravates COPD hypoxia by regulating inflammatory factors such as VEGF and TGF-β [2,16], leading to a vicious cycle of “inflammation, hypoxia and inflammation therefore, COPD inflammation and hypoxia interact with each other and promote disease progression. In addition, HIF-1α also amplifies the inflammatory effect of COPD through the regulation of inflammatory factors. The long-term inflammatory stimulation of COPD patients injures the bronchus, lung parenchyma and pulmonary blood vessels, airway remodeling, and airflow limitation, eventually leading to the reduction of FEV1% and the impairment of lung function [17].This study also confirmed that HIF-1α was negatively correlated with FEV1% in COPD patients, and the correlation showed an increasing trend with the severity of the disease, indicating that HIF-1α and Hs-CRP synergistically cause airflow limitation and impaired lung function in patients, and jointly promote the deterioration and progression of COPD. Therefore, the detection of serum HIF-1α and Hs-CRP concentrations in COPD patients can help clinicians preliminarily predict the severity of the disease and provide theoretical basis for clinical judgment of the prognosis of the disease. In the future, the sample size will be expanded to further explore the cut-off values of serum HIF-1α and Hs-CRP concentrations to predict the severity of the disease, to provide better help for clinical diagnosis and treatment of disease.
Foundation Project
a) Key Project of Natural Science Foundation of Ningxia, No.
NZ16217.
b) Key research and development project of Ningxia Hui
Autonomous Region, No.2018BEG03077.
c) Supported by school-level scientific research projects of
Ningxia Medical University, No. XM2020026, No. XM2021090,
No. XM2021092.
d) Scientific Research Project of Health System of the
Autonomous Region, No.2021-NW-061.
e) Suzhou Synergy Healthcare Foundation, No. KY-079.
f) Project of Yinchuan Science and Technology Plan
(No.2019-ZD-004, No.2021-SF-001).
g) Project of Ningxia Science and Technology Innovation
Leading Talent (No.2021GKLRLX03.
h) Project of Ningxia Health Appropriate Technology
Promotion (No.2022-NWSY-020).
References
- Lijun Chen, Wang Xu, Wenxiu Sun, Huifang Zhang, Juanxia Chen, et.al, (2021) The expression and significance of hypoxia inducible factor-1α in patients with acute exacerbation of COPD. Open Access Journal of Pulmonary & Respiratory Sciences 6(1): 000138.
- Chen Li-Jun, Chen Juan-Xia, MA Li-ting, ZHANG Hui-fang, et.al (2021) Differential analysis of HIF-1α expression in COPD and healthy people. Ningxia Med J 43(3): 229-231.
- Wu Jie, ZHANG Rui, Schwann, ZHANG Shao-Hua, Chen Li-Jun (2021) Correlation between hypercoagulable state and levels of HIF-1 α, EPO, RBC and HGB in rats with chronic obstructive pulmonary disease. Journal of Ningxia Medical University 43(6): 603-607.
- (2014) Expert Group on Diagnosis and Treatment of Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD). International J Respiratory 34(1): 1-11.
- Ding Ning, WANG Sheng (2016) Recent advances in the pathogenesis of chronic obstructive pulmonary disease. J Clin Pulmonal (1): 133-136.
- Ding Ming-Jing, XU Gui-hua, GAO Xiao-Yu, SUN De-Jun (2019) Research progress on the pathogenesis of chronic obstructive pulmonary disease. World Update Medical Information Digest (Continuous electronic Journal) 19(22): 118-123.
- Zhao J, Shi YH (2010) Role of hypoxia-inducible factor-1 and its target genes in malignant progression of tumors. Modern Oncology Med 18(1): 166-168.
- Guo Wei-Qian, ZuO Xiu-ping, ZHANG Mei, XIA Jian-wen, BAI Hua (2021) The changes and clinical significance of serum HIF-1α level in patients with chronic obstructive pulmonary disease. Western Medicine 33(11): 1622-1627.
- Song YY (2017) Expression and positive feedback mechanism of HIF-1α and MUC5AC in epithelial cells of chronic obstructive pulmonary disease [D]. Zhengzhou University
- Wang Kun, GUO Sijia, FENG Lihong, SUN Zeng Tao (2021) Research progress on common complications of chronic obstructive pulmonary disease treated by traditional Chinese medicine. Western Chinese Medicine 34(3): 149-152.
- Prins HJ, Duijkers R, van der Valk P, Schoorl M, et.al (2019) CRP-guided antibiotic treatment in acute exacerbations of COPD in hospital admissions. Eur Respir J 53(5): 1802014.
- Mattered AM, Esheba NE, Elshora OA, Mohamed WS (2019) Mean platelet volume and neutrophil to lymphocyte ratio in prediction of early diabetic nephropathy in type 2 diabetics. Clinical Research & Reviews 13(2): 1469-1473.
- Dong CH, Wang ZM, Chen SY (2018) Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: A systematic review and meta-analysis. Clin Bio chem 52: 131-136.
- Ma L, Zeng A, Chen B, Chen Y, Zhou R. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with systemic lupus erythematosus and their correlation with activity: A meta-analysis. International Immunopharmacology 76: 105949.
- Celikbilek M, Dogan S, Ozbakır O, Zararsız G, Kücük H, Gürsoy S, et.al (2013) Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal 27(1): 72-76.
- Wang Wen-Xiang, ZHANG Niu-niu, Zeng Xian-yan, et.al (2018) Effect of HIF-1 α on myocardial ischemia-reperfusion inflammatory injury [J]. Chinese Journal of Pathophysiology 34(7): 1201-1205.
- Lv Qian, WANG Chang Ming, JIANG Ming, et al (2012) Expression of HIF-1α and VEGF in rats with chronic obstructive pulmonary disease and their relationship with pulmonary vascular remodeling. Chinese Pharmacology Bulletin 28(6): 772-777.