Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1644
Review ArticleOpen Access
Leishmaniasis of the Penis: A Review and Update of the Literature Volume 2 - Issue 4
Anthony Kodzo-Grey Venyo*
- Department of Urology, North Manchester General Hospital, M8 5RB Lancashire, United Kingdom
Received: October 17, 2019; Published: October 29, 2019
Corresponding author: Anthony Kodzo-Grey Venyo, Department of Urology, North Manchester General Hospital, M8 5RB Lancashire, United Kingdom
DOI: 10.32474/OAJRSD.2019.02.000145
Abstract
Leishmaniasis is caused by Leishmania parasites that are transmitted through bites of over 90 infected species of the female
phlebotomine sand-fly. Three main forms of leishmaniasis including cutaneous, mucocutaneous, and visceral leishmaniasis exist.
The disease tends to affect some of the world’s poorest people. An estimated 700,000 to 1 million new cases and 20,000 to 30,000
deaths occur globally due to the disease. 24,200 deaths were attributed to leishmaniasis in 2015 and 4 million to 12 million cases
of leishmaniasis have been documented to exist globally. Leishmaniasis of the penis (LOP) is extremely rare. LOP does present
with subcutaneous nodules of the penis, ulceration of penis, crust on penis, erythematous scaling plaque, crusty scar on penis.
Clinical examination may reveal on the penis/glans penis: papules, crusts, serous or sero-haemorrhagic fluid secretion, usually
painless ulcer(s), hyperkeratotic plates with induration of the penis or haemorrhagic crust lesion on the penis of a child or adult.
Smears from the ulcer could typically demonstrate amastigotes, leishmania may be grown in culture of smears or specimens of
the penile lesion. Histopathology features of LOP would show a granulomatous infiltrate containing some leishmania parasites.
Histopathology examination of biopsies of the penile lesion may show ulceration of epidermis and a diffuse inflammatory infiltrate
in the dermis composed of lymphocytes, histiocytes, plasma cells, giant cells with granuloma formation and presence of leishmania
amastigotes histiocytes that contain small oval organisms with bar shaped paranuclear kinetoplast.
Haematoxylin and Eosin as well as Giemsa staining may be sufficient but with regard to positive staining for LOP, weigert ion
haematoxylin could stain better than H&E or Giemsa stain and immunostaining with G2D10 antibody tends to be more sensitive
in diagnosing LOP. The diagnostic methods available for the detection of LOP include: Intradermal delayed hypersensitivity test
(Montenegro); Culture on NNN agar using smears from the ulcer; ELIA; DNA probes; PCR. The Identification of parasites on H & E
or smears, by culture on specialized media, fluorescent antibody tests using patient’s serum, or by PCR using specific primers. But
polymerase chain reaction (PCR) results can be false positive, leishmanin intradermal skin test (Montenegro), positive culture for
leishmania from specimen or smear, zymodeme analysis and monoclonal antibody to detect leishmaniasis. A high index of suspicion
is required to undertake the most appropriate investigation to diagnose LOP. Resistance to various anti-leishmaniasis medicaments
exist in various parts of the world therefore treatment of LOP should include utilization of recommended medicament appropriate
to the geographical area of the world and could either be
a) antimoniate oral medication (doxycycline and others),
b) topical ointment (paromomycin and methylbenzethonium chloride,
c) intramuscular injection (meglumine).
With early diagnosis, appropriate treatment, and follow-up assessment, cases of LOP should resolve and this should be followed
up by appropriate steps to prevent leishmaniasis including use of nets impregnated with sand fly repellent and environmental
spraying methods to ensure control of vectors of the disease.
Keywords: Leishmaniasis; penis; Sand fly; Haematoxylin and Eosin; Giemsa stain; Novy-McNeal-Nicolle medium; meglumine antimoniate; Montenegro test; polymerase chain reaction
Introduction
Introduction
Introduction
Introduction
Leishmaniasis is a protozoan infection that is encountered in all continents globally with the exception of Australia and the Antarctica. [1] It has been stated that 1 million to 2 million new cases of various types of leishmaniasis are encountered globally per year. [1] Cutaneous leishmaniasis is an infectious disease which is caused by protozoa of the gender Leishmania, which is transmitted by stings/bites of female insects of the Phlebotomus gender in the Old World and Lutzomia within the New World. [2] It has been stated that greater than twenty species of Leishmania that is pathogenic for human beings and other mammals have been identified globally. [2,3] The clinical manifestations of leishmaniasis would depend upon the interaction between the typical virulence of the species of leishmania and the immune response of the host [2,4] It has been stated that within America, cutaneous leishmaniasis is caused by many species of the Leishmania brasiliensis complex and Leishmania Mexicana. [5-7] Cutaneous leishmaniasis which is caused by many species of protozoa belongs to the family of Trypanosomatidas, that are flagellate protozoa which live within the tissues and within the blood of the human host. [8] The vector of leishmaniasis is the sand fly and most commonly it is Phlebotomus papatasii and the commonest reservoirs include rodents, dogs, and cats. [8] The incubation period tends to be variable and this does range between 1 week to 12 weeks and the mean incubation period is 9 weeks. [8] It has been stated that cutaneous leishmaniasis has been caused by five species of Leishmania including Leismania major, Leishmania tropica, and Leishmania aethiopia, which do cause Old World leishmaniasis (in non-America countries), [8,9] and Leishmania Mexicana complex and Leishmania brasiliensis complex which are responsible for causing new-world leishmaniasis (Leishmaniasis in the Americas).
Cutaneous leishmaniasis may be treated by means of utilization of cryotherapy, localized heating, as well as utilization of Leshcutan TM ointment. [8,10] Severe cases of cutaneous leishmaniasis could be treated by means of systemic treatment with utilization of antimonial compounds, ketoconazole, allopurinol, dapsone, levamisole, and immunotherapy. [8,9] Even though between 1 million to 2 million cases of leishmaniasis are reported each year globally, Leishmaniasis of the penis is extremely rare in view of this it would be envisaged that majority of clinicians globally within Leishmaniasis non-endemic and Leishmaniasis endemic regions would not have encountered the disease before and they would therefore probably not be familiar with the presentation, investigation procedures and management of the disease. A highindex of suspicion would be required in order to establish a quick, succinct, and accurate diagnosis of the disease through undertaking of appropriate investigations to be able to provide the most appropriate management of the disease armed with the knowledge of the medicament sensitivity pattern of leishmaniasis within their localities. The ensuing review article on leishmaniasis of the penis is divided into two parts
(a) Overview and
(b) Miscellaneous narrations and discussions from some
reported cases and case series as well as studies on leishmaniasis
of the penis.
Aim
To review the literature on leishmaniasis of the penis specifically and leishmaniasis in general.
Method
Internet data bases were used including Google; Google Scholar, and PUBMED. The search engines used included: Leishmaniasis of the penis, and Leishmaniasis. Thirty-one references were identified and found suitable to write the review article.
Results / Literature Review
Overview
Definition: Leishmaniasis is a terminology that refers to a disease that is caused by parasites of the leishmania type and the disease is spread by certain types of sand flies [10] Leishmaniasis can present in three different ways including cutaneous, mucocutaneous, and visceral leishmaniasis. [10,11] Cutaneous leishmaniasis does present with skin ulcers, papules, skin encrustations, erythema or induration of the skin; the mucocutaneous form of leishmaniasis does present with ulcers of the skin, mouth, and nose; visceral leishmaniasis does start with ulcers of the skin which is ensued by fever, low red blood cell count, and hepato splenomegaly [10-12].
General Comments on Leishmaniasis
The ensuing summations have been made about Leishmaniasis
in general. [1]
• Leishmaniasis is a protozoan infection that is encountered
in all continents globally with the exception of Australia and the
Antarctica. [1]
• It has been documented that leishmaniasis in human
beings is caused by more than 20 species of Leishmania [11]
• It has been stated that more than 90 species of sand fly
have been known to transmit the Leishmania parasites. [13,14]
• Documented risk factors for the development of
leishmaniasis do include poverty, malnutrition, deforestation,
and urbanization. [11]
• All three types of leishmaniasis could be diagnosed by the
visualisation of the leishmania parasites under the microscope.
[13]
• Visceral leishmaniasis could be diagnosed by means of
blood test. [12]
• It has been iterated that about 4 million to12 million
people are infected with leishmaniasis globally currently
[13,15] in some 98 countries. [12]
• It has been documented that 1 million to 2 million new
cases of various types of leishmaniasis are encountered globally
per year [1].
• It has also been iterated that between 20 thousand and 50
thousand deaths occur globally each year due to leishmaniasis
[13,14].
• It has been stated that about 200 million people residing
in Asia, Africa, South and Central America, and Southern European countries dwell in areas where leishmaniasis is
common [12,13].
• It has been iterated that the frequency of leishmaniasis
globally is between 4 million to 12 million [10.13,15]
• It has been stated that globally in 2015 there were 24,200
deaths due to leishmaniasis [13,16].
• It has been documented that only a small fraction of
individuals that have been infected by Leishmania parasites
would develop the disease eventually [13].
• It has been stated that leishmaniasis could occur in
a number of animals including dogs, and rodents. [10,13]
Furthermore, it has been documented that some 70 animal
species, including human beings, had been found as natural
reservoir hosts of the leishmania parasites [13].
• It has been iterated that epidemics of leishmaniasis do
occur from time to time within tropical areas of the world as
well as leishmaniasis infections do afflict HIV positive patients
[1]
• It has been stated that leishmaniasis does occur generally
in various parts of the body and it does produce crusted,
indurated papule which tends to enlarge slowly and also tends
to be self-limited; nevertheless, leishmaniasis also on other
occasions does manifest as a fatal systemic illness [1].
• It has been documented that the World Health
Organization has obtained discounts on some medications to
treat leishmaniasis [12,13].
• It has been iterated that leishmaniasis is classified as a
neglected disease [1,13]
• Three sub-types of leishmaniasis have been documented
which include the following: cutaneous leishmaniasis, mucocutaneous
leishmaniasis, and visceral leishmaniasis [1].
• Considering that the clinical features of leishmaniasis
are not specific to the disease, leishmaniasis generally tends
to mimic other diseases which can be regarded as differential
diagnoses of leishmaniasis. Some of the differential diagnoses
of leishmaniasis include: Sarcoidosis; foreign body reaction;
Granulomatous Rosacea; Granuloma annulare. [1] At times
leishmaniasis lesion may mimic a malignant tumour.
Cutaneous leishmaniasis.
• It has been documented that afflictions of cutaneous
leishmaniasis tend to commonly involve the face, the scalp, the
arms and other externally exposed parts of the body [1].
• It has been stated that cutaneous leishmaniasis could be
(a) a localized disease,
(b) disseminated disease which does occur in the situation
if the immune system of the patient does not respond to the
presence of the invading leishmania parasite,
(c) recurrent cutaneous leishmaniasis which is also called
Recidivans Cutaneous Leishmaniasis, and (d) or post Kala azar disease which tends to be a rare disease and does occur years
pursuant to the development of visceral leishmaniasis [1].
• It has been iterated that cutaneous leishmaniasis is the
most common form of leishmaniasis which does cause skin
lesions which tend to be mainly ulcers, upon exposed areas
of the body which tend to leave life-long scars and serious
disability [13].
• It has been stated that approximately 95% of cutaneous
leishmaniasis do occur in the Americas, the Mediterranean
region, the Middle Eastern countries, and Central Asia [13].
• It has been documented that in 2015 more than two
thirds of new cases of cutaneous leishmaniasis had occurred
in 6 countries including; Afghanistan, Algeria, Brazil, Colombia,
Iran, (Islamic Republic of Iran), and Syrian Arab Republic [13].
• It has been iterated that it had been estimated that between
600,000 to 1 million new cases of cutaneous leishmaniasis do
occur globally each year [13].
Mucocutaneous Leishmaniasis.
• It has been documented that mucocutaneous
leishmaniasis is usually regarded as a new world disease which
does afflict individuals of Rural areas and Jungle areas [1].
• It has been iterated that muco-cutaneous leishmaniasis
does occur in situations when primary Leishmania Braziliensis
infection becomes a disseminated disease with dissemination
to the upper respiratory tract, with emanations of lesions of
oral mucosa, pharyngeal mucosa, or anal mucosa, resulting on
ulceration of the mucosa, mutilation or sometimes death of the
individual [1].
• It has been stated that mucocutaneous leishmaniasis
tends to lead to partial or total destruction of the mucous
membranes of the nose, throat, and the mouth and that more
than 90% of cases of mucocutaneous leishmaniasis do occur
in Bolivia (the Pluri-national state), Brazil, Ethiopia, and Peru
[13].
Visceral Leishmaniasis.
• Visceral leishmaniasis is also referred to as Kala Azar. It has
been stated that in cases of visceral leishmaniasis, the leishmania
parasites disseminate throughout the reticuloendothelial
system of the patient and this does lead to the development
of fever, general malaise, enlargement of liver and spleen,
anorexia, pancytopenia, and hypergammaglobulinemia [1].
• It has been documented that in Visceral leishmaniasis or
Kala azar, the skin is usually spared of disease with the exception
of the development of irregular areas of dark pigmentation for
which the terminology Kala Azar which means black sickness
had been coined [1].
• Visceral leishmaniasis could be so severe that if it is not
treated it could lead to the death of the patient [1].
• Visceral leishmaniasis has been divided into
(a) old world disease that has included: tropical and subtropical
Asia, India, Africa, and Mediterranean region; and
(b) New World which includes the Americas. [1]
• Old World – Within the old world Leishmania tropica
complex is the sub-type of leishmania disease that is
encountered [1].
• New World – Within the new world, Leishmania
Brasiliense’s Complex, and Leishmania Mexicana Complex are
the sub-types of Leishmania encountered. [1]
• It has been stated that Leishmania Brasiliense’s could also
produce espundia which is a destructive mucocutaneous form
of leishmania. [1]
• It has been documented that human infection by
leishmania does occur by means of a bite of Phlebotomus Sand
fly and on rare occasions human infection can occur through
blood contamination [1]
• It has been stated that the clinical presentation of
leishmaniasis and prognosis of leishmaniasis is variable and
it depends upon the species of leishmania causing the disease,
the duration of infection, and upon the immune status of the
individual patient. [1]
• It has been stated that leishmania infection with regard
to the United States of America primarily occurs through global
travel and HIV infection. [1]
• It has been stated that visceral leishmaniasis if left alone
untreated becomes fatal in over 95% of cases. [13]
• It has been stated that majority of cases of visceral
leishmaniasis do occur in Brazil, East Africa, and in South East
Asia. It has also been documented that an estimated 50,000 to
90,000 new cases of visceral leishmaniasis do occur each year
globally. [13]
• It has been documented that in 2015, more than 90%
of new cases of visceral leishmaniasis that had been reported
to the World Health Organization (WHO) had occurred in 7
countries including: Brazil, Ethiopia, India, Kenya, Somalia,
South Sudan, and Sudan. [13]
Presentation of Leishmaniasis of penis
A patient who has LOP may present with [17]
• Painless lump or nodule which had been present for few
weeks to many months or years.
• Induration of the penis.
• Papule on the penis.
• Crusty eruption / eruptions on the penis
• Ulceration of the penis
• Serous discharge from lesions on penis.
• No pain associated with the penile lesions.
• Most often there would be no problem associated with
coitus
• When the penile lesion is large the lesion could be
interfering with coitus.
• LOP could affect a child or an adult therefore any of the
aforementioned would present in a child or adult.
• Patient could have been healthy and on no medication
prior to the presentation.
• A history of living in or having lived in a leishmaniasis
endemic zone or travelled from a leishmania endemic zone may
be helpful to make a diagnosis.
• Usually the lesion on the penis may be the only lesion but
it is possible there may be a cutaneous lesion elsewhere.
Clinical examination findings [17]
• Generally the general and systematic examinations of the patient would tend to be normal but lesions would be found on the glans, foreskin, or any other part of the penis which may include: erythema, papule(s), ulceration, encrustation, serohaemorrhagic crusts on the penis of varying sizes depending upon the size of the lesion. It is also possible that there may be a cutaneous lesion elsewhere on the body, but this would tend to be rare.
Investigations
Urine
• Urine analysis as well as urine culture and sensitivity are general examinations that are carried out routinely as part of the general assessment of the patients, but the results would not be diagnostic of the disease/infection of leishmaniasis. [17]
Blood tests
Haematology [17]
• Full blood count, coagulation screen and erythrocyte sedimentation rate are general tests that are under taken as part of the general assessment of patients but usually the results would be normal but occasionally the erythrocyte sedimentation rate may be raised and the eosinophil count could be raised due to the fact that LOP is an emanation of a parasitic disease but the results would not be diagnostic of LOP. [17]
Biochemistry
• Serum urea and electrolytes, blood glucose, liver function tests and C-reactive protein are general tests that are undertaken but the results would not be diagnostic of LOP. The results generally would tend to be normal but rarely the C-reactive protein level may be raised. [17]
Microscopy features
• It has been stated that microscopy examination of tissues
that contain leishmania tend to show the following features:
• Dermal granulomatous inflammation with prominent
lymphocytes. [1]
• Histiocytes that contain small oval organisms with bar
shaped paranuclear kinetoplast. [1]
Diagnosis
The various ways by which leishmaniasis can be diagnosed
include:
• Intradermal delayed hypersensitivity test (called
Montenegro test); Culture on NNN agar using smears from
the leishmania ulcer; ELISA; DNA probes; polymerase chain
reaction (PCR). [1]
• Identification of parasites based up on microscopy
examination of Haematoxylin and Eosin stained specimens of
the tissue, or in smears; or by culture on specialized media;
by leishmanin intradermal skin test (Montenegro test);
fluorescent antibody tests using the patient’s serum, or by PCR
with utilization of species specific primer but it has pointed out
that PCR results could be false positive. [1]
Positive stains
• Weigert iron haematoxylin-It has been iterated that Weigert iron haematoxylin could stain leishmania parasites better than haematoxylin and eosin or Giemsa stain. [1] • Immunohistochemistry staining with G2D10 antibody has been stated to be more sensitive in comparison with Haematoxylin and Eosin in the detection of leishmaniasis. [1]
Diagnosis specific to Leishmaniasis of the penis.
• The diagnosis of cutaneous leishmaniasis and
leishmaniasis of the penis have been summated as follows: [18]
• The diagnosis of cutaneous leishmaniasis has been
frequently made clinically. Nevertheless, the demonstration of
parasites with utilization of laboratory methods is pertinent to
the establishment of a diagnosis. [19-20]
• The demonstration of amastigotes upon Giemsa staining
of smears that had been taken from the edge of lesions
represents the simplest and most efficacious diagnosis of the
disease. [19,20]
• The illustration of plasma cells and Leishmania donovani
within macrophages in the dermis by means of histopathology
examination of specimens of the lesion represents another
diagnostic tool that is utilized. [21]
Treatment
• There are many treatment options for the management
of leishmaniasis globally but resistant problems have emerged
therefore clinicians would need to rely upon the sensitivity
pattern of the area of the world to effectively treat leishmaniasis
sub-type that is involved; however, some of the available
medications or treatments include: [17]
• Meglumine antimoniate and Sodium stiboglunate.
• Amphotericin B.
• Pentamidine.
• Pentavalent antimonial
• Miltefosine
• Fluconazole
• Ketoconazole
• Itraconazole
• Levamisole
• Allopurinol
• Doxycycline
• Paromomycin
• Azithromycin
• Dapsone and Rifampicin
• Doxycycline
• Immunotherapy/Immunomodulation
• Mucocutaneous and diffuse cutaneous leishmaniasis
could be refractory to treatment. [1]
Treatment
• The planning and choice of treatment for leishmaniasis
is usually determined by where in the world the disease is
acquired, the species of Leishmania that is causing the disease,
as well as upon the type of leishmaniasis. [11,13]
• Some of the possible medications that are used for
the treatment of visceral leishmaniasis include: liposomal
amphotericin B, [13] a combination of pentavalent antimonials
and paromomycin, [11,13] and miltefosine. [22]
• With regard to cutaneous disease, paramomycin,
fluconazole, or pentamidine could be effective. [13]
Prevention
• It has been iterated the leishmaniasis could be partly
prevented by sleeping under nets that had been treated with
insecticides [11]
• It has also been stated that the spraying of insecticides to
kill Sand flies as well as treating people who have leishmaniasis
early to prevent further spread of leishmaniasis would tend to
prevent further spread of the disease. [13]
• Other documented ways of preventing leishmaniasis
include:
• Effective disease surveillance. [13]
• Control of animal reservoir hosts which is complex and
needs to be tailored according to the local situation of the
county. [13]
• Social mobilization and strengthening partnerships through mobilization and education of the community with
regard to effective behavioural change interventions. [13]
Differential diagnoses
• Some of the differential diagnoses of leishmaniasis of the
penis include: [17]
• Squamous cell carcinoma of penis.
• Lymphoma of the penis may present as a painless ulcer on
the penis.
• Haemophilus ducreyi infection of penis (a fastidious
gram-negative coccobacillus bacterial infection which is
characterized by painful sores or ulcers of the penis unlike
leishmaniasis of the penis in which the ulcers generally are not
painful).
• Syphilitic ulcers (chancres) of the penis which occur
during the primary stage of the disease about 21 days after
the initial exposure to Treponema pallidum, the gram-negative
spirochete bacterium responsible for syphilis. The chancres are
generally painless just as leishmaniasis ulcers of the penis are
also generally painless.
• Granuloma inguinale which is a genital ulcerative
disease that is caused by gram negative bacterium Klebsiella
granulomatis that used to be called Calymmatobacterium
granulomatis. The ulcers in the genital region including the
penis most commonly are painless which would simulate
leishmaniasis of penis or glans penis which is also most
commonly painless.
• Traumatic ulcer of penis.
• Viral infection of penis including Herpes genitalis and
infections that are caused by human papillomavirus (HPV)
which can cause genital ulceration, with evidence that many
infections tend to asymptomatic similar to leishmaniasis of the
penis.
• Other Bacterial infection of penis (non-specific and
specific infections including tuberculosis of the penis.
• Fungal infection of penis including candida infection
which can affect the glans penis and foreskin.
• Parasitic infection including cutaneous larval migrans
caused by hookworm infection.
• Other malignancies of penis including sarcoma, basal cell
carcinoma, melanoma, and adenocarcinoma.
• Auto-immune bullous diseases of the penis including
localized bullous pemphigoid of childhood as well as squamous
cell carcinoma of penis with bullous pemphigoid masquerading
as lymphogranuloma venereum.
• Immune and systemic diseases affecting the penis
• Drug reactions including fixed drug eruptions to penis
due to oxcarbazepine, and ornidazole.
• Inflammatory diseases of the penis.
• Papulo-squamous diseases affecting the penis including
psoriasis, Reiter syndrome, lichen planus, lichen nitidus,
seborrheic dermatitis, secondary syphilis, fixed-drug eruption,
erythroplasia of Queyrat, plasma cell balanitis of zoon,
Bowenoid papulosis, discoid and lichenoid dermatosis of
Sulzberger and Garbe.
Outcome
If visceral leishmaniasis is left not treated, most patients who
have the disease would tend to die because of the disease but
patients who receive full treatment would be expected to recover.
[17] With regard to cutaneous leishmaniasis, very few cases have
been reported in the literature and following treatment the trend
has been improvement/cure of the disease. One individual noticed
great improvement but was lost to follow-up. [17]
(B) Miscellaneous narrations and discussions from some
reported cases, case series and studies.
Cabello et al. [2] reported 2 patients who were treated for
leishmaniasis of the penis as follows:
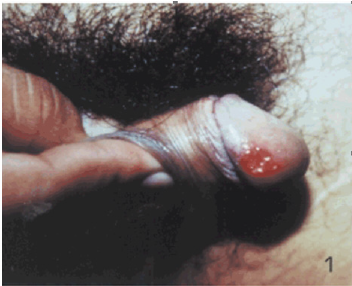
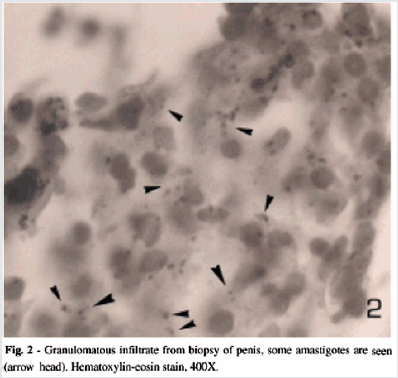
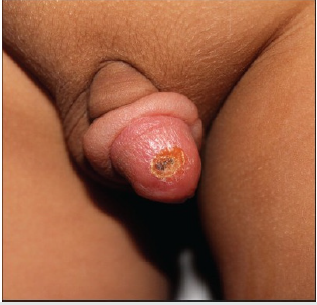
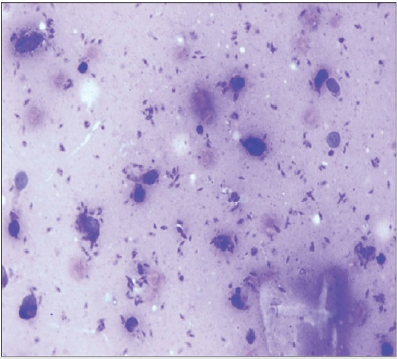
Case 1: An [17]-year old gentleman from Venezuela, had presented with a 3-months history of an ulcer on his glans penis for which he was treated by means of 2.4 million international units of intramuscular injection of penis. The ulcer did measure 1 cm and it had an indurated base as well as infiltrative borders (Figure 1). There no evidence of regional lymph node enlargement. His Venereal Disease Research Laboratories (VDRL) and Fluorescent Treponemal Antibody Absorption test results were negative. He had Montenegro’s intradermal test and the was positive and it had yielded an indurated papule that measured 2.6 cm. Microscopy histopathology examination of the lesion did reveal ulceration of the epidermis as well as a diffuse inflammatory infiltrate within the dermis which had comprised of lymphocytes, histiocytes, plasma cells, and giant cells that had formed a granuloma and additionally the examination did show presence of Leishmania amastigotes (Figure 2). He was treated by means of meglumine antimoniate intramuscularly (20mg of Sb+/kilogram per day) for three weeks. The lesion did heal fully at the end of the treatment [2].
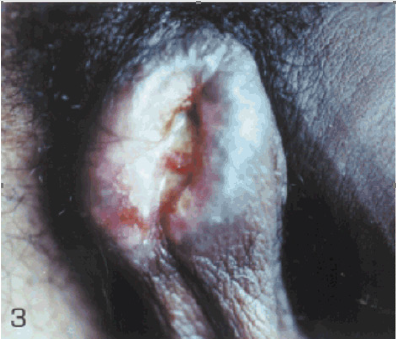
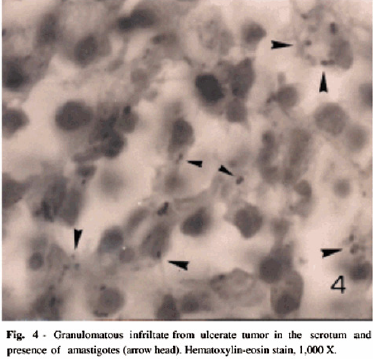
Case 2: Cabello et al. [2] reported a 38-year-old Venezuelan man who had been suffering from a tumour in his scrotum of 5 months duration for which a provisional clinical diagnosis of squamous cell carcinoma was made. His clinical examination did reveal an ulcerated tumour that measured 5cm x 3cm with regard to diameter and which had an indurated base that was raised. The ulcerated lesion also had inflammatory borders and from the lesion was noticed a yellowish exudate (Figure 3). He had Leishmania skin test and the result was positive. The results of his HIV (enzymelinked immune-absorbent assay [ELISA], and VDRL were negative. Histopathology examination of a specimen taken from the lesion showed pseudoepitheliomatous hyperplasia and a necrotic area as well as ulceration of the epidermis that was associated with a diffuse inflammatory infiltrate within the dermis that was comprised of lymphocytes, plasma cells, polymorphonuclear cells and vacuolated histiocytes with the presence of Leishmania amastigotes (Figure 4).
He was treated by means of meglumine antimoniate intramuscularly (with a dose of 20mg of Sb+ per kilogram per day for three weeks and this resulted in complete cicatrisation of the lesion. Cabello et al. [2] stated that within the preceding years there had been an increase in the number of cutaneous leishmaniasis within the Bolivia state, and Venezuela. A lesson that needs to be learnt from the aforementioned two case reports is that the incidence of Leishmaniasis infection of the penis though rare has been on the increase in Bolivia and Venezuela, therefore, clinicians within Bolivia and Venezuela would need to have a high index of suspicion for the disease in order to establish a quick accurate diagnosis of the disease in order to provide a quick and accurate appropriate treatment for the disease. Additionally, globally, clinicians should be aware of the possibility of Leishmaniasis of the penis if patients travel from certain areas of Bolivia and Venezuela to other parts of the world if they present with unusual penile lesions. These two cases have illustrated that 3-weeks daily intramuscular injections of meglumine antimoniate is effective for the treatment of Leishmaniasis of the penis and the scrotum [2].
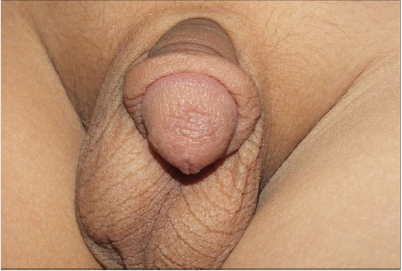
Gülüm et al. [23] had reported a 45-year-old man who did present with a crusty scar on his penis which he had noticed for a period of approximately 4 years. He was asymptomatic otherwise. He did not have any significant past medical history apart from having been having coital difficulties over a period of one year preceding his presentation. His clinical examination revealed that he had hyperkeratotic plates which had fully spread all over his glans penis and which had a slight indurated base and sometimes serohaemorrhagic crust lesion and fissure (Figure 5). The results of his routine blood tests were normal. He had leishmaniasis smear test which was normal (Figure 6). He did have excisional biopsies taken from the hyperkeratotic lesion and histopathology examination of the specimens did exclude benign and malignant skin conditions. He received treatment by means of intralesional meglumine antimoniate twice weekly for 8 eight weeks and after which the treatment was continued with utilisation of 20 milligrams per kilogram of meglumine antimoniate. Over the period of treatment, an improvement was observed in the hyperkeratotic plates and shrinkage in the penile lesion had also been noted. There was a further plan to continue is treatment with the use of 20 milligrams per kilogram of meglumine antimoniate but unfortunately the patient subsequently failed to attend for follow-up assessment and treatment. This case is pertinent to clinical practice in that some lesions of the penis might look like malignant penile lesions but could turn out not to be malignant and knowledge that leishmaniasis is a differential diagnosis should make it possible for clinicians have a high-index of suspicion for leishmaniasis of the penis so that they can decide early to undertake a leishmania smear test. Giemsa (Figures 5,6) have been reproduced from: Gülüm M [23].
Figure 5: A non-healing hyperkeratotic plates, which fully
spread in the glans penis.
Reproduced from [23]. The Copyright is maintained by the
Journal and copyrights would need to be obtained from
the Journal for any further use.
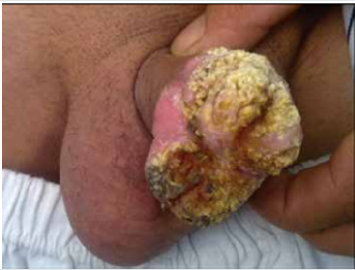
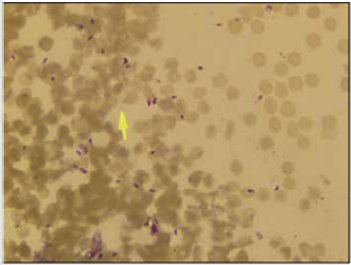
Figure 6: The typical presentation of amastigotes in a
smear preparation, stained with Giemsa.
Reproduced from [23]. The Copyright is maintained by the
Journal and copyrights would need to be obtained from
the Journal for any further use.
Schubach et al. [24] did report a man who had presented with an ulcerated lesion of his glans penis that had later on been followed by the development of mucosal lesions of his nasal cavity and palate. He did have investigations including leishmania skin test (Montenegro’s test) and biopsy of the border of the ulcer which had been submitted for histopathology examination and culture in Novy-McNeal-Nicole (NNN) medium. The results of the tests had revealed the following:
• The leishmania skin test was positive.
• Histopathology examination of the specimen showed a
granulomatous infiltrate that contained some parasites.
• Culture of the specimen was positive for leishmania
SP- and this was subsequently identified by utilisation of
zymodeme analysis and monoclonal antibodies as Leishmania
(V.) Braziliensis.
• With regard to medical treatment, he did receive
pentavalent antimony at a dose of 10 milligrams per kilogram
per day for 30 days which did result in healing of the lesions.
Schubach et al. [24] made the ensuing summations.
• With regard to men, particularly those who are older than
50 years of age, ulceration of the glans penis would tend to be
highly suggestive of carcinoma of the penis.
• Precise differential diagnosis of penile ulcer is imperative
with regard to the establishment of the correct diagnosis.
• A lesion such as the one in the case they had reported,
could pose diagnostic difficulties when it does manifest in
countries that are different from the source of infection, where
the condition is very uncommon.
• In view of the current era of widespread global travel the
finding of leishmaniasis of penis has been frequently increasing.
This article is useful in that it would illustrate to clinicians in countries where leishmaniasis is not common that leishmaniasis of the penis can occur on rare occasions; therefore, clinicians should be alert and should consider leishmaniasis as a differential diagnosis of penile ulcers and to undertake tests that would confirm or exclude leishmaniasis of the penis.
Grunwald et al [25] had reported a 30-year-old man who had manifested with a lesion on his glans penis that had been present over the preceding 2 months. His clinical examination revealed an erythematous scaling plaque which had measured 15 mm in diameter. His general and systematic examinations were normal. A smear was that taken from the lesion which had shown large macrophages that were filled by many amastigotes of Leishmania which are called Donovan bodies. He was treated by local application of paromomycin and methylbenzethonium chloride ointment (Leshcutan TM, Teva, Israel). He noticed was marked improvement of the lesion. Yesilova et al. [18] had reported a 5-year-old boy who did present with a 6-months history of an asymptomatic, and persistent ulcer on his penis which had been treated by utilization of topical steroids, anti-bacterial agent, anti-scabetic agent, antiviral preparations; nevertheless, none of the treatment approaches had been successful. He did not have any family history of similar symptoms. His clinical examination revealed a 2.0cm x 3.0cm ulcer that had a haemorrhagic crust on his glans penis (Figure 7). There was a mild painless, bilateral inguinal lymph node enlargement. The results of his routine haematology and biochemistry blood tests and C-reactive protein as well as his erythrocyte sedimentation rate were noted to be normal. The results of the following tests were also normal or classed as negative:
• Venereal Disease Research Laboratory (VDRL) test.
• Fluorescent Treponemal Antibody Absorption (FTA-ABS)
test.
• Serological tests for herpes simplex virus, cytomegalovirus,
Epstein-Barr Virus (EBV), Chlamydia Trachomatis, Human
Immunodeficiency Virus.
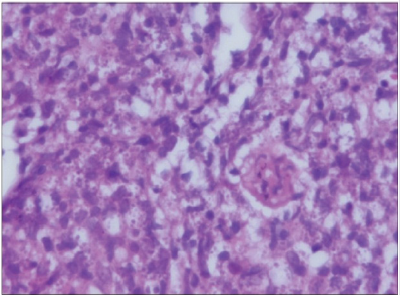
Figure 9: Histopathology of the skin punch biopsy showing
numerous microphages distended with amastigotes (H&E,
x 1000).
Reproduced from [18].
Examination of Giemsa stained smear obtained from his lesion did reveal amastigote forms that had been suggestive of Leishmaniasis (see figures 8 and 9). Promastigotes forms of the parasite had been found in the Novy-McNeal-Nicole (NNN) culture medium. Histopathology examination of biopsy specimens of his lesion had revealed features which were compatible with the diagnosis of cutaneous leishmaniasis. With regard to treatment, he had received two doses of intramuscular injections of meglumine antimoniate with a dose of 20 milligrams per kilogram weight, 20 days apart. He did not develop any complications. At his 20- day follow-up appointment, it was observed that his penile lesion had appeared to be healing with a mild scar tissue (Figure 10). This case report has been important in that it had illustrated that leishmaniasis of the penis can occur in children and also that meglumine intramuscular injections given at 20 days interval for children is effective in the treatment of leishmaniasis of the penis affecting a child (Figures 8,9) [18]. Herbert et al. [26] had reported a 75-year-old Caucasian man who had presented with a severe recalcitrant ulcer on his glans penis which he had developed over a period 8 months preceding his presentation. He underwent circumcision one year earlier for phimosis because of a sequel of lichen sclerosus with a good post-operative unremarkable recovery. However, several months after his circumcision he developed penile ulcer that had continued to increase in diameter despite his use of various topical anti-inflammatory and anti-infectious treatments.
He did not have any significant past medical history and there was no discharge from the lesion. His clinical examination showed an inflammatory crusty ulcer on his glans penis which had measured 2.5cm in diameter. Based upon the history and clinical examination finings the provisional differential diagnoses that were made did include: Erythroplasia Queyrat, squamous cell carcinoma of the penis, and balanitis circumscripta plasmacellularis zoon. His systematic examination was unremarkable except for a painless enlargement of the left inguinal group of lymph nodes. Microbiology cultures of swabs that were taken from the ulcer and crust did not reveal a growth of any fungus or bacterium. The results of his routine haematology and biochemistry blood tests were within normal range except for a slightly elevated C-reactive protein level. The result of his syphilis serology (VDRL) was normal. He did have biopsy of the border of the lesion and histopathology examination of the specimen did show complete ulceration of the epithelium and granulomatous infiltrate which had contained multinucleated giant cells, T lymphocytes and plasma cells. Microscopy examination of the haematoxylin and eosin stained specimen of the biopsy was inconspicuous for infectious causes of granulomatous diseases, especially amastogote leishmania parasites. Examination of the biopsy specimen that had been stained by PAS and Ziehl-Neelson staining were found to be negative for any specific infection. However, the polymerase chain reaction (PCR) test result was positive for Leishmaniasis. Herbert et al. [26] stated that their results did confirm an earlier statement that polymerase chain reaction (PCR) could be useful for the diagnosis of leishmaniasis, particularly with regard to the scenario in which the leishmania parasite load is low.
Masmoudi et al. [28 ] had reported a 41-year old man who had presented with 20 days history of nodular lesion on the anterior aspect of his neck, 2 juxta-apposed ulcerated nodular lesions on the left wrist, and subcutaneous nodules which had been arranged linearly and had extended to the root of his penis. The lesions were covered by an erythematous or ulcerated skin. Examination of smears which were obtained from the genital lesions of the penis did confirm the diagnosis of cutaneous leishmaniasis. The evolution of the disease became favourable following 21 days treatment with doxycycline after an interval of 1 week. The case report of Masmoudi et al. [27] is important for two reasons
(a) it had illustrated that leishmaniasis of the penis can also
occur as part of multiple lesions on some occasions and not as a
single spot lesion localized to the penis as reported sporadically.
(b) doxycycline which is an inexpensive medication which is
taken orally as well as which is globally available is efficacious in
the treatment of leishmaniasis of the penis.
Castro Coto et al. [28] had reported 5 cases of leishmaniasis in the genital organs of males who were farmers. Four of the five men were aged less than 30 years and one of the patients was older than 30 years. All five patients were residents of high leishmaniasis incidence zones.
Most of the lesions did affect the penis, and the lesions were of scabies ulcer type, destructive, painless, and of slow evolution. Castro Coto et al. [28] iterated that with regard to the five cases, generally, the clinical diagnosis of leishmaniasis was difficult to corroborate by means of the habitual laboratory methods as well as they further stated that the principal differential diagnoses of leishmaniasis of the penis are neoplastic processes of the genital area. They also stated that a high index of suspicion for the diagnosis of leishmaniasis of the penis would be required even before initiating the most appropriate diagnostic/therapeutic tests that would provide the solution to this type of diagnostic dilemma. The aetiopathogenesis of ulcers of the penis includes: trauma, viral infection, bacterial infection, fungal infection, parasitic infection, malignancies, auto-immune bullous diseases, immune and systemic diseases, drug reactions, inflammatory diseases, and papulo-squamous diseases. [18] However, majority of the ulcers of the penis tend to be caused by sexually transmitted diseases that include syphilis, genital herpes virus infection, lymphogranuloma venereum, and donovanosis. [18] Additionally, it has been stated that non-venereal infections including candida, Epstein Barr virus, bacteria, parasitic, and mycobacterial infections could emanate information of ulcers of the penis [18].
Furthermore, it has been documented that Syphilitic chancres tend to be painless and they also tend to be persistent in comparison with ulcers and vesicles which had emanated from genital herpes which tend to be painful as well as to be associated a high degree of inflammation. [19,29] It has also known that Haemophilus ducreyi does cause soft chancre, that is characterised by painful ulcers of the penis and tend to be associated with tender lymph node enlargement [18]. With regard to non-steroidal anti-inflammatory medications, it had been documented that sulphonamides, penicillin, tetracyclines, anti-epileptic medicaments, and anti-malarial medications could also be responsible for the causation of erosive as well as ulcerative drug reactions in the genital mucosa. [18] It has been stated that inflammatory and papulosquamous diseases inclusive of psoriasis, lichen sclerosus, angiokeratomas, lichen nitidus, lichen planus, behnet’s disease, Reiter’s syndrome, pyoderma gangrenosum, pemphigus, as well as inflammatory bowel disease could also affect the penis. It has been iterated that neoplastic diseases which cause ulcers of the penis do include; erythroplasia of Querat, squamous cell carcinoma, basal cell carcinoma, extramammary Paget’s disease, lymphoma, and leukaemia and malignant diseases that could cause ulcers of the penis. [18] It has been documented that on rare occasions, ulcers of the penis which are related to mechanical trauma, chemical trauma, thermal trauma, and factitial reasons could also be encountered. [29-31]
Conclusion
Leishmaniasis of the penis is an uncommon treatable disease which can affect children as well as adults and could easily be missed as a diagnosis in view of its rarity and the fact that globally majority of clinicians would not have encountered the disease and would not be familiar with the clinical manifestations of the disease. Leishmaniasis is endemic in few countries in all continents of the world except Australia and Antartica. Leishmaniasis is endemic in only few countries in the world but because of increasing global travelling these days clinicians should be aware that they could encounter various types of leishmaniasis including leishmaniasis of the penis any time without being notified. Leishmaniasis of the penis should be regarded as a differential diagnosis of all the common lesions of the penis by having a high index of suspicion for the disease so that a history of travels should be obtained from all patients who do present with ulcerative and crusty penile lesions and they should quickly undertake all the necessary investigations to exclude a diagnosis of leishmaniasis of the penis if the diagnosis is not obvious.
Acknowledgements
Acknowledgement to
• The Turkish Journal of Parasitology (Turkiye Parazitol
Derg) for allowing figures taken from an article in the Turkish
Journal of Parasitology (Turkiye Parazitol Derg) under CC By-NCND
licence to be reproduced for the single use of this article. The
copy right is still maintained by Turkish Journal of Parasitology
(Turkiye Parazitol Derg) and any further reproduction of the
figures would require copy right permission from the Turkish
Journal of Parasitology (Turkiye Parazitol Derg).
• The Indian Journal of Dermatology, Venereology and
Leprology for permitting reproduction of contents and figures
taken from under Copy Right Permission: This is article is
available under the terms of the Creative Commons Attribution-
NonCommercial Share Alike License (CC By-NC-SA), which
permits non-commercial use, distribution and reproduction
in any medium, provided the original work is properly cited.
Permission only needs to be obtained for commercial use.
• Rev. Inst. Med. Top. S. Paulo for granting copy right
permission for reproduction of figures from their journal under
CC BY-NC All the contents of their Journal, except otherwise
noted, is licensed under a Creative Commons Attribution
License.
References
- Hamodat M (2018) Skin inflammatory (non tumour) infectious disorders Leishmaniasis PathologyOutlines.com last major update 2011.
- Cabello I, Caraballo A, Millán Y (2002) Leishmaniasis in the Genital Area Rev. InstMed Top S Paulo 44(2): 105 -107.
- Ridley DS (1980) A histological classification of cutaneous leishmaniasis and its geographical expression. Trans Roy Soc Trop Med Hyg74: 515 - 521.
- Pearson RD, Sousa AQ (1996) Clinical spectrum of leishmaniasis. Clin InfectDis 22: 1-13
- Cupolillo E, Grimaldi Jnr G, Momem H (1994) A general classification of new world Leishmaniasis using numerical zymotaxonomy. Amer J Trop Med Hyg50: 296-311.
- Grimaldi Jr G, Tesh RB, McMahonPratt D (1989) A review of the geographic distribution and epidemiology of leishmaniasis in the new world. Am J Trop Med Hyg41: 687-725.
- Grimaldi Jr G, Tesh RB (1993) Leishmaniasis in the new world: current concepts and implications for future research. Clin Microbiol Rev 6(3): 230-250.
- Grunwald (1998) Amichal, trau Cutaneous leishmaniasison an unusual site --- the glans penis British Journal of Urology 82: 928.
- Arnold HL, Odom RB, James WD (1990) Parasitic infections, stings, and bites. In Arnold HL, Odom RB, James WD,(eds), Andrew’s Disease of the skin, (8th) Philadelphia: W B Saunders486: 92.
- Leishmaniasis Wikipedia https://en.m.wikepedia.org Accessed 2018 Nov 13
- Leishmaniasis Fact Sheet. N”375” World Health Organization 2014 Jan.
- Barrett M P, Croft S L (2012) Management of trypanosomiasis and leishmaniasis British Medical Bulletin 104: 175-196.
- (2018) WHO Leishmaniasis – World Health Organization.
- Lozano R (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859): 2095-2128.
- (2016) GBD 2015 Disease and injury Incidence and Prevalence, Collaborators, Global, regional, and national incidence, prevalence with disability for and years lived with disability for 310 diseases and injuries /, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053): 1545-1602.
- (2016) GBD 2015 Mortality and Causes of Death, Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053): 1459-1544.
- Venyo AKG (2019) Leishmaniasis of the Penis: A Review and Update. Jacobs Publishers.
- Yeslova Y, Turan E, Sürücü HA, Kocarslan S, Tanrikulu O, et al. (2014) Ulcerative penile Leishmaniasis in a child. Indian J Dermatol Venereol Leprol 80(3): 247-249.
- Gurel MS, Yesilova Y, Olgen MK, Ozbel Y (2012) Cutaneous leishmaniasis in Turkey. Turkiye Parazitol derg 36(2): 121-129.
- Kocarslan S, Turan E, Ekinci T, Yesilova Y, Apari R (2013) Clinical and histopathological characteristics of cutaneous Leishmaniasis in Sanliurfa City of turkey including Syrian refugees. India J Pathol Microbiol 56(3): 211-215.
- Pehoushek JF, Quinn DM, Crum WP (2004) Cutaneous leishmaniasis in soldiers returning from deployment to Iraq. J Am Acad Dermatol 51(5): 197-200.
- Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ (2012) Miltefosone: a review of its pharmacology and the therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67(11): 2576-2597.
- Gülüm M, Yeşilova Y, Savaş M, Ciftҁi H, Yeni E, et al. (2013) A Case of Giant Hyperkeratotic Cutaneous Leishmaniasis in the Penis. Turkiye Parazitol Derg 37(1): 53–54.
- Schubach A, Cuzzi-Maya T, Conҁalves-Costa SC, Pirmez C, Oliveira-Neto MP (1998) Leishmaniasis of Glans Penis. J Eur Acad Dermatol Venereol 10(3): 226-228.
- Grunwald, Amichal, trau (2002) Cutaneous leishmaniasison an unusual site - the glans penis. British Journal of Urology 82(6): 928-928.
- Herbert YG, Boer-Auer A, Brandenburg A, Falk TM, Reiter-Owona I (2015) Severe ulcerative penile leishmaniasis – Importance of PCR-based diagnostics. Journal of Infection 71(1): 139-141.
- Masmoudi A, Boudaya S, Bouzid L, Frigui F, Mezoiu TJ, et al. (2005) Penile sporotrichoid cutaneous leishmaniasis. Bull Soc Pathol Exot 98(5): 380-381.
- Castro Coto A, Hidalgo Hidalgo H, Solano Aguilar E, Coto Chacón F (1987) Leishmaniasis of the genital organs. Med Cutan Ibero Lat Am 15(2): 145-150.
- Bulat V, Situm M, Ljubicic I, madiraca D (2012) Wounds in the genital and oral region. Acta Med Croatica 66(1): 109 -117.
- Teichman JM, Sea J, Thompson IM, Elston DM (2010) Noninfectious penile lesions. Am Fam Physician 81(2): 167-174.
- Arya M, Kalsi J, Kelly J, Muneer A (2013) Malignant and premalignant lesions of the penis. BMJ 346: 1149.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...