
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1373
Research ArticleOpen Access
Furosemide Diuretic Activity Evaluation in Female Sprague-Dawley Rats Volume 1 - Issue 4
Luis Castillo Henríquez1,2*, Andrea Campos Cerdas1,3 and Rebeca Chacón Durán1,4
- 1Pharmacy Faculty, University of Costa Rica
- 2Phytopharmacology and Pharmaceutical Technology Laboratory (LAFITEC), Institute of Pharmaceutical Research (INIFAR), Costa Rica
- 3Regulatory Affairs, Calox, Costa Rica
- 4Pharmacy department, Coopesalud RL, Costa Rica
Received:August 16, 2019; Published: August 22, 2019
Corresponding author: Luis Castillo Henríquez, Phytopharmacology and Pharmaceutical Technology Laboratory (LAFITEC), Institute of Pharmaceutical Research (INIFAR), San Jose, Costa Rica
DOI: 10.32474/LOJPCR.2019.01.000119
Abstract
This research article focuses on the evaluation of the diuretic activity of Furosemide administered to a group of female Sprague Dawley rats, through the use of metabolic boxes.
Methods: A hydration volume of 5 mL/100 g was administered by cannulas for oral administration to group I (Furosemide) and to group II (control). After 2 h the basal urine volume was determined. Group I was given 20 mg/kg of Furosemide and group II 20 mg/kg of physiological saline solution, both intraperitoneally. After the administration of the new hydration load of 5 mL/100 g, the measurements of excreted urine volume and its pH were done every 30 minutes for 2 h.
Results: The maximum urinary excretion caused by this drug was achieved at 30 minutes post - administration, which shows a statistically difference with respect to the control group. However, after 60 minutes it is observed that the diuretic activity begins to decrease markedly due to the compensatory mechanisms of the physiology of the animal and the pharmacokinetics of the drug.
Conclussion: The rapid onset of action and brief effect showed by Furosemide is consistent with what is established in the literature. Likewise, the slight decrease in post-treatment urinary pH with respect to the basal level, adjusts to the effect of Furosemide itself and to the expansion effect of the urinary volume.
Keywords: Diuretic Activity; Furosemide; Intraperitoneal Administration; Loop Of Henle; Metabolic Boxes; Sprague-Dawley; Urine Excretion
Abbreviations: ANP: Atrial Natriuretic Peptide; BNP: Brain Natriuretic Peptide; NKCC: Sodium-Potassium-Chlorine cotransporter; SD: Sprague-Dawley
Introduction
Alterations in the balance and regulation of liquids and electrolytes are very common. Excess fluids and solutes are usually corrected through the use of diuretics, which are described as a heterogeneous group of drugs that perform their function in different segments of the nephron. They block the reabsorption of water and solutes, promoting the excretion of the liquid by increasing the urine flow [1,2]. One class of this pharmacological group is represented by the loop diuretics, such as Bumetadine, Etacrynic Acid, Torsemide and Furosemide. They are closely linked to albumin, so they must undergo secretion in order to have access to the site of action in the tubular lumen. Secretion can be prevented if there are high levels of endogenous organic acids or by drugs that share the same transporter [3]. Loop diuretics are part of the first-line treatment in systolic and diastolic insufficiency, where sodium and fluid retention are very common. Therefore, they are frequently used in order to remove fluids. This class of drugs such as Furosemide, blocks sodium reabsorption and provides symptomatic relief of volume overload, improves exercise tolerance and avoids hospitalization. They are also important in the immediate reduction of congestion and pulmonary edema associated with acute heart failure [4,5]. Furosemide is the most commonly used loop diuretic and, with respect to others, it has the most significant pharmacokinetic profile, so its use is very advantageous. Its mechanism of action consists in the inhibition of Na+ and Cl- reabsorption in the thick ascending loop of Henle and in the distal tubule, by causing a reduction of the potential difference, which generates an increase in the excretion of H2O, Na+, Cl-, Mg+2 and Ca+2 [3,4,6]. The Na+ K+ 2Cl- (NKCC) cotransporter inhibitors are chemically a diverse group. Particularly, Furosemide is a sulfonamide derivative. This type of loop diuretics has a short-term diuresis, after the administration of the dose and reaches its action in the apical membrane not only by filtration but also by secretion of the nephron. A full dose generates a massive diuresis of Cl- and Na- if the glomerular filtration rate is normal [7]. The present study aims to evaluate the diuretic activity of Furosemide, administered intraperitoneally to a group of female Sprague-Dawley (SD) rats, compared to a control group for which a physiological saline solution was given.
Materials and Methods
Materials
SD female rats (200-250 g), 1 mL syringes, 10 mL graduated cylinder, cannulas for oral administration, physiological saline solution, metabolic boxes and the pHmeter were provided by the Pharmacy Faculty of the University of Costa Rica. Furosemide 1 g/ mL ampoules were provided by a national industry.
Methods
Eight female SD rats were divided into two groups and both
were subjected to light / dark cycles of 12 h/12 h duration.
They remained with ad libitum access of water and food during
pretreatment. A total volume of hydration of 5 mL/100 g of the
animal was administered through a cannula for oral administration.
After that, they were allowed to rest for two hours in their respective
metabolic box and the basal level of urine volume and its pH were
recorded at the end of this period. Next, the following treatment
administered intraperitoneally was performed to each group:
a) Group I: 20 mg/kg of Furosemide.
b) Group II (control): 20 mg/kg of physiological saline solution.
For the determination of the excreted urine volumes, the rats
were placed back in the metabolic boxes and urine volumes were
recorded at 30, 60, 90 and 120 minutes after the administration of
either the drug or the physiological saline solution.
The diuretic action was calculated by the following formula:
Diuretic action=(Average volume excreted by the Furosemide
group)/(Average volume excreted by the Control group)
The statistical analysis of the data was performed using the
SPSS15 Statistical Software.
Results
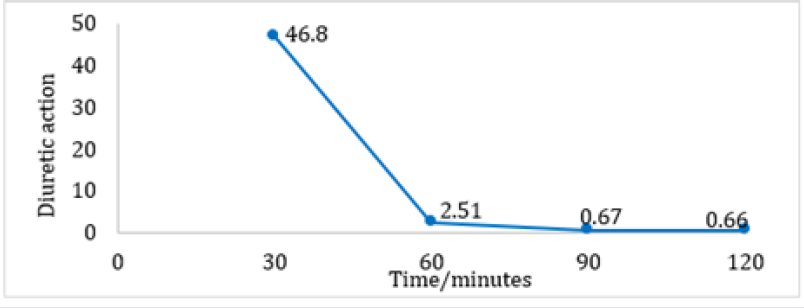
A significant difference exists in the average volume of excreted urine between the Furosemide and control group at 30 and 60 minutes. Also, within the Furosemide group there is a statistical significance regarding the excreted volumes at 30, 90 and 120 minutes. It is important to mention that the basal urine volume levels for both groups showed close average values (Furosemide: 4,70 mL and Control: 4,95 mL). Regarding the average percentage volume of excreted urine for Furosemide group, the maximum values are obtained at 30 and 60 minutes. On the other hand, at 120 minutes no volume of excreted urine was obtained. However, at 90 minutes the control group reported an average percentage volume of excreted urine superior to the one of the group that was given the drug, indicative that the diuretic effect at that time was almost none It is quite notorious that Furosemide showed a rapid onset and reached its maximum diuretic action at 30 minutes. After that, the action falls abruptly until constant values at 90 and 120 minutes (Table 1). As can be seen in (Figures 1-4), the group that was given Furosemide almost excreted a 100 % of the liquid administered at 120 minutes, while the control group barely excreted a bit more than half of the volume.
Table 1: Variation of the average pH of the excreted urine after the pretreatment and post-treatment for group I and II.
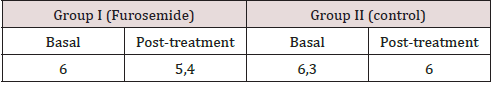
Figure 1: Average volume of excreted urine after the administration of 20 mg/kg of Furosemide or physiological saline solution at 30 minutes intervals.
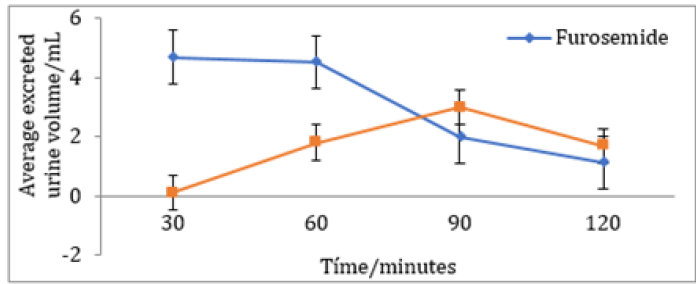
Figure 2: Average percentage volume of excreted urine after the administration of 20 mg/kg of Furosemide or physiological saline solution at 30 minutes intervals.
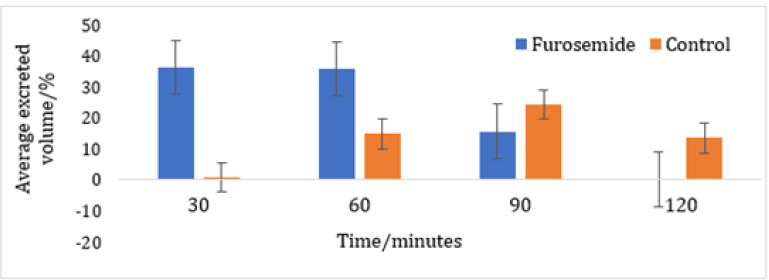
Figure 3: Accumulated average percentage volume of excreted urine after the administration of 20 mg/kg of Furosemide or physiological saline solution at 30 minutes intervals.
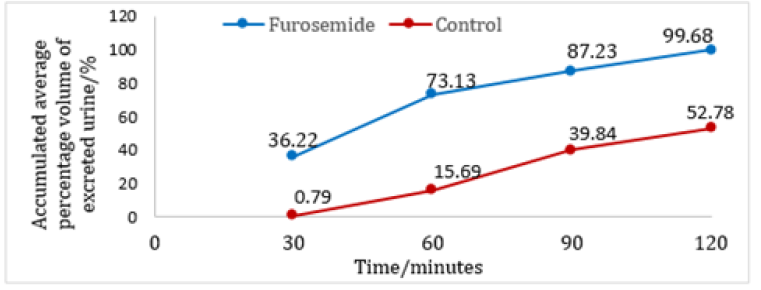
Figure 4: Diuretic action of Furosemide evaluated in a group of female SD rats at 30 minutes intervals.
Discussion
Furosemide is considered as a high ceiling diuretic, since it is very effective and powerful compared to other diuretics that act beyond the thick ascending limb, where only a small percentage of filtered sodium reaches [2]. The results observed in (Figure 3) show that the diuretic effect of Furosemide is short, but with a rapid onset of action. Regarding its pharmacokinetic characteristics, the half-life has to be found in a range of 0.5 to 2 hours, depending on the renal function. The time required to observe the diuretic effect produce by the intravenous administration is around 5 minutes [8]. However, given the difficulty of using the intravenous route in small animals, intraperitoneal administration was considered the best option. The results obtained show a behavior similar to those that would be obtained through the intravenous, since both routes of administration have a great bioavailability [9]. The increase in diuresis due to the administration of Furosemide can be explained by the inhibitory effect of the drug on the NKCC cotransporter, which acts in the thick ascending portion of the loop of Henle, producing an elevantion in the concentration of NaCl and KCl at the level of the lumen of the nephron, which leads to an increase in osmotic pressure and produces a decrease in water reabsorption [10].
The reason why Furosemide has a rapid onset of action can be due to the fact that its secretion in the proximal tubule is effective by the organic acid transport system, so it is easier to block the NKCC cotransporter [2,8]. The effective circulating volume remains regulated thanks to the sympathetic nervous system and the reninangiotensin- aldosterone system. The dense macula detects the concentrations of NaCl leaving the loop of Henle and regulates the release of renin depending on the concentration of Cl- and Na+ ions in the lumen [11]. Likewise, by decreasing intravascular volume, vasopressin is released in baroreceptors, which senses the drop in blood pressure and activates autonomic nerve pathways, such as the ones directed to the hypothalamus area and where vasopressin release is controlled. In the collecting duct, the insertion of the water channels is stimulated increasing the number. Therefore, greater water absorption is produced [12]. The maximum urinary excretion in the control group (90 min) occurs by compensatory mechanisms like the action of the atrial natriuretic peptide (ANP) and to a lesser extent the brain natriuretic peptide (BNP) which are secreted in the heart, mainly by the atrial muscle cells. The secretion granules increase in number, causing the secretion of both peptides when the atria and ventricles dilate respectively. This is mainly due to an increase in NaCl intake and/or an increase in extracellular volume. The two peptides, produce natriuresis and increased urine by dilating the afferent arteriole and relaxing the mesangial cells. In addition, they inhibit sodium reabsorption in the renal tubules, renin secretion and do not allow the effects of catecholamine pressure and angiotensin II [13]. As seen in the results, group I and II, showed a slight decrease in urine pH. The above is due to the expansion of the aqueous urinary volume, which gives the activation of the sodium-hydrogen-ATPase antiporter, producing a hydrogen secretion and passive bicarbonate reabsorption. In the case of the Furosemide group, occurred an exchange of sodium and chlorine for hydrogenation in the collecting tubule, causing a slightly more acidic pH [14].
Conclusion
The diuretic action of Furosemide and the diuretic activity profile observed are consistent with what is established in the literature, where it is indicated that this drug has a rapid onset of action and a short effect. In addition, the slight decrease in posttreatment urinary pH with respect to the value measured in basal urine adjusts to the effect of the expansion of the urinary volume and that of Furosemide itself. Finally, as a recommendation, in order to obtain better results, the amount of S.D rats for the control group and for the treatment with the drug should be increased to obtain more statistically significance in the results. Similarly, urine collection intervals could be reduced after drug administration, in order to know the exact time at which the maximum diuretic action occurs. It is also recommended to use analytical methods, such as spectrophotometry, to determine the concentration of ions present in the urine.
References
- Francey T (2015) Chapter 160-Diuretics. In: Small Animal Critical Care Medicine (2nd). WB Saunders USA, pp. 846-850.
- Carone L, Oxberry S, Twycross R, Charlesworth S, Mihalyo M, et al. (2016) Furosemide. JPSM 52(1): 144-150.
- Miles J, Hanumanthu B, Patel K, Chen M, Siegel R, et al. (2019) Torsemide versus furosemide and intermediate-term outcomes in patients with heart failure. Journal of Card Medicine 20(6): 379-388.
- Tan V, Nash D, McArthur E, Jain A, Garg A, et al. (2017) Impact of Injectable Furosemide Hospital Shortage on Congestive Heart Failure Outcomes: A Time Series Analysis. Canadian Journal of Cardiology 33(11): 1498-1504.
- Matsue Y, Damman K, Voors A, Kagiyama N, Yamaguchi T, et al. (2017) Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. Journal of American Coll Cardiology 69(25): 3042-3051.
- Cil O, Haggie P, Phuan P, Tan J, Verkman A (2016) Small-Molecule Inhibitors of Pendrin Potentiate the Diuretic Action of Furosemide. JASN 27(12): 3706-3714.
- Lan C, Peng C, Tang S, Lin H, Yang S et al (2017) Inhibition of Na-K-Cl cotransporter isoform 1 reduces lung injury induced by ischemia-reperfusion. Journal of Thorac Cardiology Surgery 153(1): 206-215.
- Magallanes L, Fgiolino P, Vazquez M, Fotaki N, Ibarra M, et al. (2016) Sex-related in vitro/in vivo and PK/PD correlations after oral single dose furosemide administration. Journal Pharmacology Technology & Drug Research 5(1): 1-11.
- Turner P, Brabb T, Pekow C, Vasbinder M (2011) Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. Journal American Association Labaratory Animal Science 50(5): 600-613.
- Lamarche C, Pichette M, Ouimet D, Vallée M, Bell R, et al. (2016) Pharmacokinetic and Dynamic of Furosemide in Peritoneal Dialysis Patients. Journal of International Social Periti Dialysis 36(1): 107-108.
- Riet L, Van Esch J, Roks A, Van Den Meiracker A, Danser A (2015) Hypertension: Renin-Angiotensin-Aldosterone System Alterations. Circ Research 116(1): 960-975.
- Lovett E, Grassi G (2015) Baroreflex Activation Therapy in Heart Failure. In: Gronda E, Vanoli E, Costea A. Heart Failure Management: The Neutral Pathways. Springer USA, pp. 183-197.
- Sato Y, Dohi K, Watanabe K, Tanimura M, Takeuchi T, et al. (2016) Combination of Urinary Sodium/Creatinine Ratio and Plasma Brain Natriuretic Peptide Level Predicts Successful Tolvaptan Therapy in Patients With Heart Failure and Volume Overload. International Heart Jornal 57(2): 211-219.
- Perinpam M, Ware E, Smith J, Turner S, Kardia S, et al. (2017) Association of urinary citrate excretion, pH, and net gastrointestinal alkali absorption with diet, diuretic use, and blood glucose concentration. Physiological Reports. 5(19): 1-10.




