Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1373
Research ArticleOpen Access
Development and Characterization of Herbal Formulation Containing Aqueous Extract of Clitoria Ternatea Linn. (Flowers) for the Treatment of Vaginal Infection Volume 2 - Issue 4
Urvashi Sharma* and Sumeet Dwivedi
- University Institute of Pharmacy, Oriental University, Indore, India
Received: April 08, 2021 Published: April 19, 2021
Corresponding author: Urvashi Sharma, University Institute of Pharmacy, Oriental University, Indore, India
DOI: 10.32474/LOJPCR.2021.02.000143
Abstract
Among several gynecological problems, vaginal infection (vaginitis) is the most common problem that women face in their life span, caused by Candida species. Candida albicans, a fungus which is responsible for the vaginal infection and to cure it, innumerable herbs are available which were earlier frequently used by the local civilization but now their demand is increasing in global market also. Numerous studies have confirmed the use of Clitoria ternatea as an antimicrobial agent, so the present work has been undertaken to explore the anti-candida activity of the plant. Along with that, formulation of herbal cream was aimed. In this study, the herbal creams (HC1-HC8) were formulated and evaluated possessing aqueous extract of flowers of Clitoria ternatea Linn. and the results revealed the satisfactory results when compared with standard drug. The results of formulation with code HC5 were found to be very promising and excellent of all herbal cream formulated. Ensuring biological activity for any formulation is of prime interest to reveal and prove the efficacy of formulated dosage form. In view of the same, Herbal Creams (PHC) were prepared and subjected to anti-candida activities, where PHC of HC5 was evaluated for anti-Candida activity as an optimized preparation. From the results obtained, it was concluded that the PHC (HC5) have optimum anti-candida activity when compared with standard formulation (MCC) and may be used for the treatment of vaginal infection. Moreover detailed clinical approaches need to establish for the formulation of safe and effective drugs
Keywords:Clitoria ternatea; Anti-fungal; Vaginal infection
Introduction
Over the course of a lifetime, 75% of all women are likely to have at least a vaginal Candida infection and up to 45% have experiences it two or more, making it the commonest gynecologic diagnosis in primary care. As women tend to be more prone to vaginal yeast infections because of several reasons such as stress, poor nutrition, poor hygiene, lack of sleep, illness, hormonal imbalance or while pregnant or taking antibiotics. Women with immunosuppressive diseases such as diabetes and HIV infection are also at greater risk [1] and This infection also known as Vaginitis is characterized by symptoms, including discharge, odor, itching, irritation, or burning (Figure 1). Often causes by Candida species especially Candida albicans that accounts for 85% to 90% of cases [2]. From the ancient era, many plants and herbs are being used to treat various infirmities; one of them is Clitoria ternatea L, commonly known as Aparajita. In all ancient scriptures of Ayurveda, it is mentioned as one of the important herb [3]. Clitoria ternatea, also known as butterfly pea, is a perennial twinning herbaceous plant which belongs to the Fabaceae family. Major phytoconstituents of the plant responsible for several activities are flavonols namely kaempferol, 7-rhamnoside, quercetin, anthocyanins such as ternatin and delphinidin and the pentacyclic triterpenoids viz. taraxerol and taraxerone. The herb is being used for the treatment of diabetes, pain, inflammation, vaginal irritation and infections and worm infections [5].
Materials and Methods
Selection of plant material
The herb Clitoria ternatea Linn. is being used for the treatment of gynecological disorders especially vaginal infection from so long by the folks but till date no systematic investigation has been carried out to formulate the effective herbal formulation using the flower alone or in combination to treat gynecological disorders (vaginitis), therefore, this herbs were selected for the research work [6-10].
Collection and authentication of plant
The plant material selected for the present investigation was flowers of Clitoria ternatea Linn. and were collected in the months of Dec 2020 to Jan 2021 from various sites of Malwa region of Madhya Pradesh and identified & authenticated by Dr. S.N. Dwivedi, Professor and Head, Department of Botany, Janata PG College, A.P.S. University, Rewa, (M.P.) The specimen was deposited in Laboratory, with Voucher specimen No. P/CT-F/1403.
Preparation and characterization of extracts [5]
Preparation of extracts:- 250 gm of the air dried coarsely powdered flowers of Clitoria ternatea Linn. was placed in soxhlet apparatus and was extracted with distilled water until the extraction was completed (till 24 hr.). After extraction, the filtrate was evaporated, and percentage extract was determined.
Characterization of extract:- The aqueous extract of dried plant material of Clitoria ternatea Linn. (flowers) CTF were characterized for their color, odor, taste and pH and were recorded in present investigation.
Anti-candida activity of extracts
Fungal strain:- Fungal strain i.e., Candida albicans (ATTC 10231) was obtained from Medical College and was used for the present investigation.
Screening of antifungal activity by disc diffusion method:-
Preparation of disc: Disc of Whatman filter paper of one quarter inch in diameter was prepared and the same was sterilized using autoclave.
Preparation of samples entrapped disc: The accurately weighed extracts were dissolved in methanol and different stock solutions (10, 20, 30, 40, 50 μg/ml) solutions were prepared. All the dilution prepared was applied to Whatman filter paper disc using a micropipette. The discs were then dried and sterilized.
Preparation of culture plate: The Sabouraund’s agar and Mueller Hinton agar media were prepared by dissolving media in 1000ml of distilled water and sterilized by autoclaving at 121oC for 1 hour. The media were cooled and poured in sterilized Petri plates to solidify at room temperature.
Collection of fungal strains: The fungal strains (Candida albicans) were obtained from Index Medical College, Malwanchal University, Indore. The inoculums of strain were transferred to the re-cultured before starting the lab work.
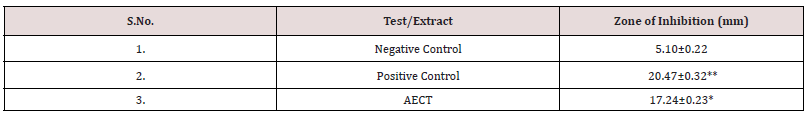
Evaluation of zone of inhibition: The re-cultured fungal strains were used for antifungal evaluation. The strains were streak on the Mueller Hinton media and the drug entrapped discs were placed. For negative control, disc of distilled water and for positive control clotrimazole disc (10 μg/ml) were used. The disc of aqueous extract of dried flower of Clitoria ternatea Linn. was prepared having 10μg/ ml concentration. The petri plates were kept in incubator for 24 hrs. After 24 hrs, the petri-plates were checked for zone of inhibition. The zone of inhibition diameter was recorded with the help of zone reader scale. The zone of inhibition was calculated by subtracting diameter of sample or standard or control by diameter of disc. The more the zone of inhibition the more will be antifungal activity.
Statistical analysis: All the reading obtained were analyzed using one way analysis of variance i.e., ANOVA. Student t-test was used. The values were found to be statistically significant (*P<0.00, **P<0.01). All the values obtained were expressed as mean± standard error means (SEM).
Formulation of herbal cream[6,7]
The herbal cream was prepared using fusion method and the various steps involved in formulation of herbal cream were as described below.
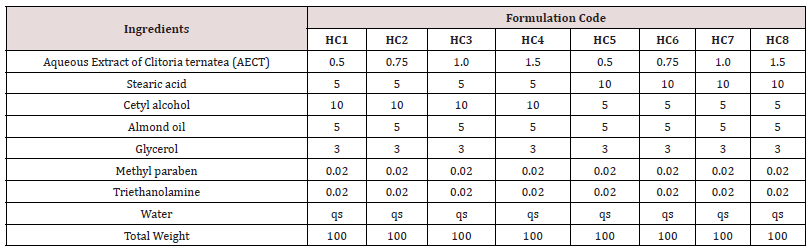
Preparation of oil phase: Stearic acid, cetyl alcohol, almond oil were taken in desired quantity in porcelain dish as per the Table 1 and were melted at 700C.
Preparation of aqueous phase: Aqueous extract of dried plant material of Clitoria ternatea Linn. glycerol, methyl paraben, triethanolamine and water as per Table 1 were taken in another porcelain dish and were heated at 700C.
Addition of aqueous phase to oil phase: The aqueous phase was added to the oil phase with continuous stirring at room temperature. Perfume was added at last and the formulation was transferred in a suitable container.
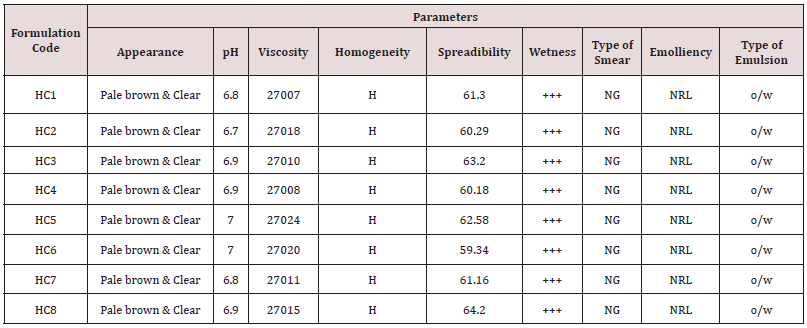
Physical evaluation: The prepared creams were evaluated for physical characteristics by evaluating clarity and transparency which was determined through visual inspection by observing the sample in light at white background (Tables 2 & 3).
Determination of pH
pH of the prepared formulations was determined using digital pH meter. The pH meter was first calibrated, and zero reading was recorded. The samples were diluted with water to make suspension and were taken in the beaker and the readings were taken from calibrated electrode. The procedure was repeated, and reading were taken in triplicate and recorded.
Determination of viscosity
The viscosity of the herbal cream was determined by Brookfield Viscometer using spindle no. 1 working at 20 rpm at temperature 4oC and 37oC. About 15ml of the samples from each batch was taken in beaker and spindle was immersed in the formulation (Figure 2). The reading was recorded at initial and after rotation at different temperature. The reading was recorded thrice.
Determination of homogeneity
Homogeneity of various formulations was tested by visual observation and reported. The formulations were also evaluated for presence of any aggregates or gritty particles (Tables 4 & 5).
Determination of spreadibility
The formulations were placed on the glass slide and the empty glass slide was place on the top of cream containing slide. The herbal cream placed between slides was pressed to form thin uniform layer. The two slides were fixed and on the upper glass slide, the weight of 20 ± 0.5 g of the weight was tied. Due to weight, both the slides were separated, and the time required to separate the two slides, i.e., the time in which the upper glass slide moved over the lower plate was taken as measure of spread ability. The three readings were recorded, and mean time was taken. The spread ability was calculated using the formula:
S= m x l/t
Where,
m = weight tide to upper slide
l = length moved on the glass slide
t = time taken.
Determination of wetness
The prepared herbal creams were determined for wetness by applying on skin surface. The amount of cream taken from each batches must be the quantity sufficient to apply at 10 mm2 area of skin surface.
Determination of type of smear
The prepared herbal cream was applied on the skin surface and after the application the type of film or smear formed on the skin was recorded. The amount of cream taken from each batches were quantity sufficient to apply at 10 mm2 area of skin surface.
Determination of emolliency
The prepared formulation was checked for emolliency, slipperiness and amount of residue left after the application of cream. To determine emollinecy, the sufficient quantity of cream was taken from each batches and applied at 10 mm2 area of skin surface and the results were noted.
Determination of Type of Emulsion
Dilution test: The prepared herbal cream was diluted with oil or water depending upon the type of emulsion whether o/w or w/o the results obtained were noted down.
Dye solubility test: The prepared herbal cream was mixed with a water-soluble dye i.e., amaranth and was observed under the microscope. The results obtained were interpreted.
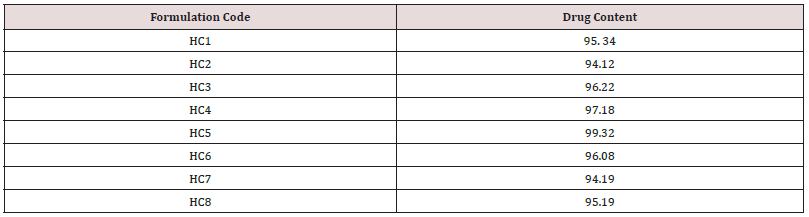
Determination of drug content: The drug content of the herbal creams was estimated using UV-Visible spectrophotometer. Nearly about 1g of the formulation was taken in 50 ml of volumetric flask and the solution was made up to mark with methanol. The solution was shaken and filtered through what man filter paper and about 0.1ml of the filtrate was further diluted to 10ml with solvent and estimated at suitable wavelength.
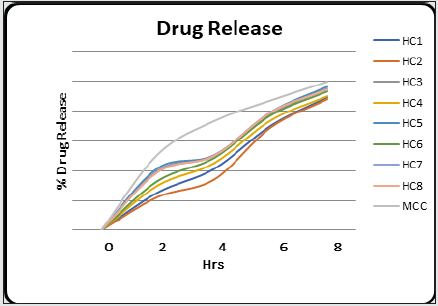
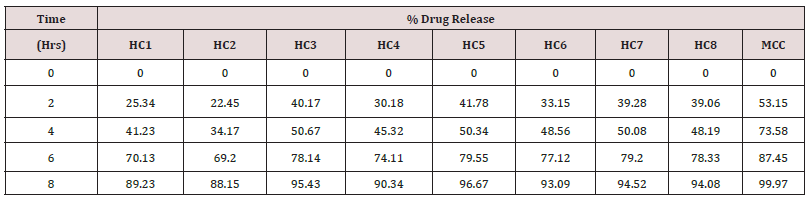
In vitro drug release/dissolution test: The semi permeable dialysis membrane bag (7cm long) was prepared, and the herbal cream was placed in the membrane. The dialysis bag was then suspended in 50ml of ethanol: water (1:1) at temperature 37°C ± 0.5°C in water bath. About 1ml of sample was withdrawn from the membrane at predetermine regular intervals (2, 4, 6 and 8 hr.) and the fresh equal volume was replaced simultaneously. The samples were withdrawn, diluted and analyzed by UV Visible spectrophotometer at suitable λmax for herbal cream containing aqueous extract of Clitoria ternatea Linn. The experiment was repeated thrice, and the cumulative drug release was calculated from the reading.
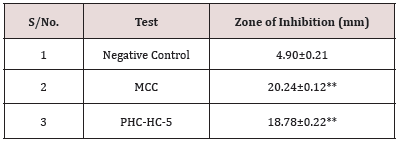
Anti-Fungal activity of optimized formulation: The antifungal activity (anti-candida activity) of optimized herbal cream and standard cream formulations was determined by disc diffusion method and results obtained for Zone of inhibition were compared. Marketed Clotrimazole cream (MCC) 1% w/w IP was used as a standard.
Results and Discussion
The herbal cream containing aqueous extract of Clitoria ternatea flowers was prepared and evaluated on various parameters. All the preparations gave satisfactory results on all the defined parameters and the results are shown in Table 2. From the results of drug content of formulated herbal cream containing aqueous extracts of dried plant material of Clitoria ternatea Linn. (Flowers) CTF and different proportion of excipients, it was found that HC5 has maximum drug content in combination therefore, HC5 were evaluated for anti-Candida activity. From the results (Table 3) obtained it was concluded that the PHC (HC5) have optimum and significant anti-candida activity when compared with standard formulation (MCC) and hence, it may be used for the treatment of gynecological disorders (vaginitis). Moreover detailed clinical approaches need to establish for the formulation of safe and effective drugs.
Conclusion
Candida albicans, a fungus which is responsible for the vaginal infection. Several literatures have suggested the effectiveness of Clitoria ternatea as antifungal agent for the treatment of vaginal infections. To prove it scientifically, anti-candida activity of aqueous extract of selected plant were evaluated. The zone of inhibition of extract on Candida albicans were investigated and results indicated that the selected extract have significant anti-candida activity when compared with standard drug Clotrimazole. From the results obtained, it was concluded that the extracts of selected herb have optimum anti-candida activity and may be used for the treatment of gynecological disorders (vaginitis) (Table 6). The results of formulation with code HC5 were found to be most promising and excellent among all herbal creams formulated when compared with standard formulation (MCC) and may be used for the treatment of vaginal infection. However detailed research study needs to be done to develop this as an effective formulation.
References
- Paladine HL, Desai U (2018) Vaginitis: Diagnosis and Treatment. American Family Physician 97(5): 321-329.
- Lopez JEM (2015) Candidiasis (vulvovaginal). BMJ Clin Evid 2: 800-815.
- Singh N, Gupta J, Shah K, Mishra P, Tripathi A, et al. (2017) A Review on Clitoria ternatea(Linn.): Chemistry and Pharmacology. Medicinal Plants and its Therapeutic Uses 1: 1-17.
- Oguis G, Gilding E, Jackson M, Craik D (2019) Butterfly Pea (Clitoria ternatea), a Cyclotide-Bearing Plant with Applications in Agriculture and Medicine. Frontier in Plant Science 10: 600-645.
- Mukherjee P, Kumar V, Kumar NS, Heinrich M (2008) The Ayurvedic medicine Clitoria ternatea-From traditional use to scientific assessment”. J Ethnopharmacol 120(3): 291-301.
- Shriwas S, Dwivedi S (2020) Development and Evaluation of Herbal Cream Containing Hydro-Alcoholic Extract of Clitoria Ternatea Linn. (Roots) Used for the Treatment of Vaginal Infection. International Journal of Womens Health and Gynecology 2(1): 110-115.
- Aulton ME (2002) Pharmaceutics: The Science of Dosage Form Design. Churchill Livingstone, London 2(5): 322-334.
- Shriwas S, Choukse R, Dwivedi S (2019) Formulation and Evaluation of Herbal Cream Containing Hydro-Alcoholic Extract of Achyranthes aspera Linn. (Roots) Used for the Treatment of Vaginal Infection. Acta Scientific Medical Sciences 3(8): 192-196.
- Saad AH, Ahmed SN, Mohamed EB (2013) Formulation and Evaluation of Herbal Cream from Ziziphus Spina Leaves Extract. International Research Journal of Pharmacy 4(6): 44-48.
- Sharma U, Sharma A, Jain NK (2020) Development and Evaluation of Anti acne Cream using Herbal Extracts of Vitex Negundo and Hibiscus Rosasinesis. International Journal of Pharmaceutical Sciences and Research 0975-8232/2320-5148: 67-72.