Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Mini Review(ISSN: 2641-1709) 
Thyroid Stunning – A Myth Or A Reality & Does It Adversely Affect The Treatment Outcome In Differentiated Thyroid Cancers? Volume 7 - Issue 4
P Shanmuga Sundaram* and Subramanyam Padma
- Clinical Professors, Department of Nuclear Medicine & Molecular Imaging, Amrita Institute of Medical Sciences & Research Centre, Amrita Vishwa vidyapeetham University, Kerala, India
Received: November 15, 2021; Published: November 30, 2021
Corresponding author: P Shanmuga Sundaram, Clinical Professor, Department of Nuclear Medicine & Molecular Imaging, Amrita Institute of Medical Sciences & Research Centre, India
DOI: 10.32474/SJO.2021.07.000270
Abstract
Radio iodine (131 I) is a well-established diagnostic and therapeutic tool that is very effective in differentiated thyroid carcinoma
(DTC) with and without metastases. Stunning of thyroid tissue is a visual phenomenon often described in post therapy 131 I
patients after undergoing 131 I whole body (WBI) diagnostic scans. It may be defined as a temporary suppression of iodine trapping
function of the thyrocytes and thyroid cancer cells as a result of the radiation given off by the scanning (or first) dose of 131 I. In
other words, there is non-visualization of 131 I concentrating thyroid remnant or distant metastases which was in fact present in
the pretherapy (diagnostic) scan. This phenomenon is still controversial, and few suggest that it could influence the efficacy of high
dose 131 I therapy, making it unsuccessful. However, the underlying mechanism still remains unclear.
Aim: Firstly, to determine the various factors that can lead to thyroid stunning in DTC patients. Secondly, is there a reduced
therapeutic efficacy of the therapeutic dose that affects the final treatment outcome.
Materials & Methods: This retrospective study was conducted in a tertiary care university hospital with data obtained from
227 DTC patients (Male: female = 34: 193, Age range: 16 – 78 yrs, Median age: 40 years). Majority of patients were papillary thyroid
carcinomas. All patients underwent thyroidectomy, diagnostic WBI scans followed by high dose 131 I therapy.
Results: Based on post therapy WBI scan findings, patients were categorized into two groups, with stunning and no stunning effect.
Patients were on regular follow up (mean 24 ± 3 months) and underwent WBI scan, serum thyroglobulin (Tg), anti Tg antibody.
Statistical analysis was done with Fischer’s exact test and 2 tailed p values were calculated. No statistically significant difference in
the treatment outcome between both groups was observed.
Conclusion: Our study concludes that thyroid stunning is indeed only a visual phenomenon encountered in 17.62% of our DTC
patients undergoing high dose 131 I therapy. It however does not affect the therapeutic outcome even in those patients who demonstrated
stunning in post therapy WBI scans substantiated by undetectable or stimulated Tg value <2ng/ml. Of the various factors
studied, it has been found that dose and timing of therapy after diagnostic I 131 scan are most important. Doses as low as 1.5 mCi
of I 131 also demonstrate stunning effect. Other factors that are found to influence the occurrence of stunning are delay between
diagnostic scan and therapy, time between treatment and post therapy WBI scan, and timing of follow-up scans.
Keywords: Differentiated thyroid cancer; 131 I whole body scan; Post therapy WBI scan; thyroid stunning
Introduction
There is evidence of increasing thyroid cancers worldwide especially in the last three decades. Rising incidence and higher detection rates of DTC is attributed to the expanding use of imaging techniques, biopsy procedures like fine-needle aspiration (FNA) and medical surveillance in the detection of small, subclinical PTCs [1]. Conventionally, 3–4 weeks after total thyroidectomy, a low dose (diagnostic) 131 I scintigraphy is performed to assess the presence of residual thyroid tissue and also to quantify the amount of remnant tissue either by thyroid uptake method or by planar imaging. In presence of residual thyroid tissue, patients undergo 131 I ablation (30-50mCi, millicuries). 131 I therapy is the term used when higher doses are used to treat metastatic foci. During the process of WBI scan and high dose 131 I treatment in post thyroidectomy setting, a phenomenon called ‘stunning’ has been encountered. As per its literal meaning, stunning means thyrocytes both benign and malignant that received a certain amount of beta radiation from a diagnostic dose of 131I was unable to trap and retain subsequent therapeutic (higher) dose of 131 I. The stunned cells may not be able to take up the ensuing therapeutic radioiodine-131 to the degree of their original unaffected capacity. It may lead to an incomplete ablation of the thyroid remnant or metastatic lesion. This phenomenon is described in patients who demonstrated 131I uptake on a diagnostic scan but absence of uptake on the subsequent post-therapy WBI scan at that site. Few studies report that stunning can not only affect the thyroid tissue in neck but can also affect metastatic sites. However, there is larger evidence that stunning can happen, at least in remnant thyroid tissue.
Materials & Methods
This retrospective study was conducted in a tertiary care
university hospital with data of patients who presented to Nuclear
medicine department from Jan 2018 – 2019. 227 DTC patients
fulfilled the selection criteria and were considered for analysis.
Selection was based on following inclusion criteria:
a) Adult patients (> 18 years of age) with histologically
proven DTC (Papillary: Follicular),
b) Never received 131 I either as a diagnostic or a therapeutic
dose.
c) No prior contrast enhanced CT studies, at least for 3-4
weeks prior.
d) Follow up WBI scan done after 6 months post therapy
with Stimulated Serum Tg, anti Tg antibodies values.
Those with any of the following were excluded from the analysis
a). Low risk DTC patients with thyroid tumor size < 1–1.5 cm
with no capsular invasion or distant metastasis.
b). Minimally invasive follicular cancer of tumour size more
4cm.
c). Patients with neither papillary nor follicular histology
subtypes who need no high dose 131 I therapy
d). Poorly differentiated thyroid carcinoma
e). Unavailable pathologic results
f). Initial diagnostic WBI scan performed elsewhere
g). Patient who did not undergo diagnostic WBI scan prior to
high dose 131 I treatment.
Surgical treatment details were obtained for all the 227 patients.
Majority of them underwent total or near total thyroidectomy (n =
126) followed by hemithyroidectomy or Completion thyroidectomy
(n = 49). 38 / 227 (16.74%) patients underwent modified neck
dissection along with total thyroidectomy. Subtotal thyroidectomy
was performed in 14 patients. Histologically 192 patients (85%)
were papillary thyroid cancers while remaining 35 patients
harbored follicular type. Mean follow up for 227 patients was 24
± 3 months.
Patient preparation
Patient preparation is important prior to WBI scan and also for subsequent 131 I residual thyroid ablation or high dose 131 I therapy. All patients should avoid iodine containing foods such as seafoods, iodized salt, drugs (cough expectorants, povidone iodine, amiodarone) and iodinated contrast agents for at least 3-4 weeks prior to the procedure. It is mandatory to stop Thyroxine prior to WBI imaging or therapy in order to increase the endogenous TSH (thyroid stimulating hormone) levels above 30 uIU/ml for successful ablation. Following 131 I ablation/therapy, post therapy 131 I whole body scintigraphy is performed 5–7 days later to ascertain whether there is optimal concentration of 131 I in the residual thyroid tissue or sites of known metastatic foci. It is also used to screen the patient for any unsuspected regional/distant metastases in order to plan further doses of 131 I therapy after 6 months.
WBI scan protocols
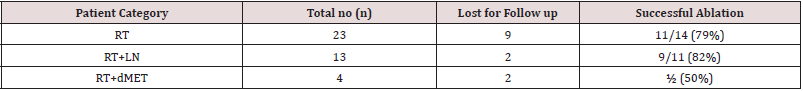
55.5 MBq, mega becqueral (1.5 mCi, millicurie) of 131 I was orally administered (in the form of solution or capsule) to patients prepared with iodine free diet, TSH > 30 uIU/ml and overnight fasting. 48 hours later high-resolution anterior neck image (in extended neck position) was acquired for quantitation purpose (Figure 1A) on a dual head variable angle Gamma camera with high energy collimators (OPTIMA NM 640 SPECTCT). This was followed by whole body 131I scan from head to toe (Figure 1B). A delayed post therapy 131I whole body scan was acquired 3 to 5 days after high dose 131 I administration (Figure 1C). Follow up 131 I scan (Figure 1D) are planned 6 months. Here 5mCi of 131 I is administered orally and WBI scan is performed 72 hours later with estimation of stimulated Tg and antiTg antibodies.
Interpretation
Focal sites of 131 I uptake are visually noted in diagnostic I 131 neck scan. Quantitative estimation of the amount of residual thyroid tissue is also undertaken for dosimetry calculations. The region of interest is drawn over the residual thyroid tissue and percentage of 131 I uptake by remnant thyroid tissue is calculated (Figure 1A). Mechanism of 131 I uptake is explained by the fact that DTC cells concentrate 131 I by functioning sodium iodine symporter, or NIS mechanism. Once the required dosage is delivered, intracellular concentration of 131 I exert a crossfire or bystander effect (selective targeted therapy) and thus destroys all the residual thyroid tissue and metastatic foci. This effect is slow and takes 5-6 months. Therefore, patients successfully treated show no 131 I uptake in neck / metastatic sites in 6 monthly scan supported by undetectable Tg and normal antiTg antibody levels. However, patients with residual thyroid and metastatic deposits will depend on disease burden and may need more than one dose of therapy for complete resolution to occur.
Figure 1: Example of a patient showing no stunning effect.
A): Anterior neck image demonstrating residual thyroid tissue. Percentage of 131 I uptake by remnant thyroid tissue is
calculated (12%) (SSN - Suprasternal notch)
B): Diagnostic WBI scan (anterior, posterior views) showing significant residual thyroid tissue with no distant metastases.
C): Post therapy WBI scan showing good concentration of I 131 uptake in significant residual thyroid tissue (arrow).
D): Follow WBI scan after 6 months shows successful ablation.
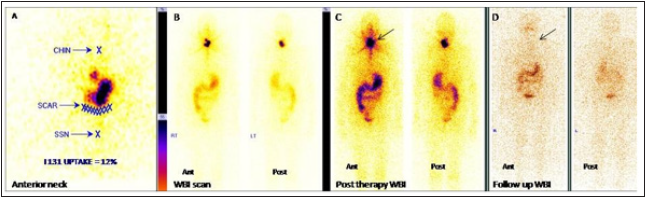
Patterns of stunning
a. Good 131 I uptake noted in thyroid bed in diagnostic
scan but no uptake in neck in post therapy scan (Figure 2) –
Complete stunning effect.
b. Uptake in thyroid bed is present but reduced when
compared to diagnostic scan (Figure 3) – Partial stunning effect.
c. Reduction in 131 I uptake in neck and metastatic sites on
post therapy scan (Figure 4).
The initial definition of stunning was expanded to include cases
in which there was reduced uptake rather than complete absence
of activity on the post therapy WBI imaging. Clinical interest in
this phenomenon is based on some investigators showing inferior
results of 131 I treatment in patients with stunning. Based on the
post therapy findings, patients were categorized into:
a) Group S (+) - patients with stunning
b) Group S (-) - patients with no stunning
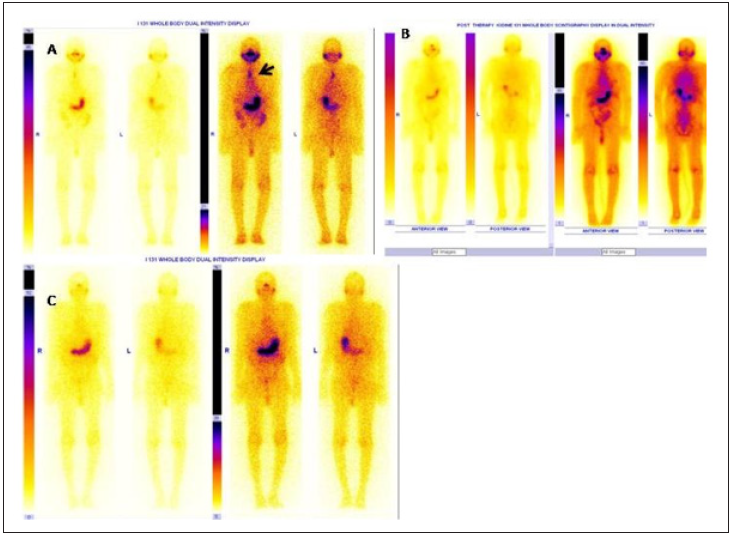
Figure 2: Example of a patient showing stunning effect in thyroid remnant tissue with successful ablation in 6 months post
therapy scan with undetectable stimulated Tg value.
A,B,C: Anterior neck, diagnostic WBI scan and post therapy WBI scan image.
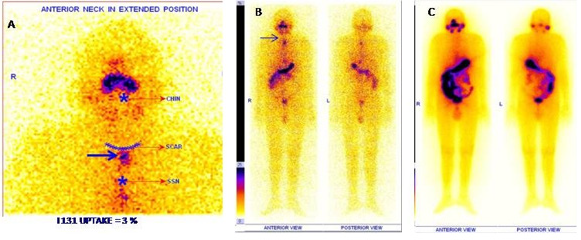
Figure 3: Example of a patient showing partial stunning effect in A: Residual thyroid tissue (bold arrow) and upper cervical
nodal metastases (dotted arrow)
B: Diagnostic WBI scan (high intensity),
C: Faint I 131 uptake in thyroid bed and cervical nodal metastases in post therapy WBI scan.
D: Follow WBI scan after 6 months demonstrating successful ablation with stimulated Tg of 0.2 ng /ml.
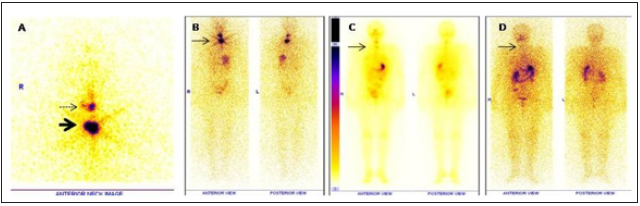
Figure 4: A: Diagnostic WBI scan in two different intensities, – demonstrating I 131 concentrating sternal metastases (bold arrow) in an elderly
male with Follicular ca thyroid with stimulated Tg of 106 ng/ ml.
B: Post therapy WBI scan after 5 days of 180 mCi of I 131 administration. No uptake noted at the site of sternal metastases ( complete stunning).
C: Follow up WBI scan after 6 months shows complete ablation of functioning sternal metastases with stimulated Tg of 3 ng/ml. Patient has
been suggested close follow up.
Results
Based on scan findings, patients were classified
a. As those with only residual thyroid tissue (RT),
b. With additional lymph nodal metastases (RT + LN)
c. With distant metastases (RT + dMET).
Patients with residual thyroid tissue (RT) received 50 - 60
mCi of 131 I as a single oral dose, while those with additional neck
nodes (RT + LN) received 100-120 mCi and patients with distant
metastases were treated with more than 150 mCi. 17.62% (40/ 227)
patients showed stunning effect during post therapy WBI scans. Of
the patients who demonstrated (S+) stunning, successful ablation
was noted in 79%, 82% and 50% in RT, RT+ LN, and RT + d MET
group (Table 1). 187/ 227 (82.38%), patients showed no stunning
effect. In the S- group the overall success rate was 91%, 71% and
44 % in the three groups of patients respectively, RT, RT+ LN, and
RT + d MET group (Table 2). Of the 40 patients who demonstrated
stunning effect, 22 displayed partial stunning and 18 exhibited
complete stunning. More number of patients with partial stunning
phenomena had successful ablation i.e, 13/15 patients (87%). 8/12
patients with complete stunning were successfully treated (66.7%)
(Tables 3 & 4). All patients underwent post therapy WBI scanning
5-7 days later. Statistical analysis was done with Fischer’s exact test
and 2 tailed p value calculated and. Outcomes were measured in
each of these patient groups and p was statistically not significant
in all three groups (p = 0.17; p = 0.71; p = 1 respectively). There
was no influence of age, sex, histological types of DTC in S+ and Sgroups
of patients. Average time interval between pre therapy WBI
scan and high dose 131 I therapy were also not significant. Time
interval between S + group and S- group were 2 weeks and 2.5
weeks respectively. Of the various factors studied, it has been found
that stunning dose and timing of therapy after pretherapy imaging
are most important.
Discussion
Entity of thyroid stunning was first reported in 1951 by Rawson
et al. [2]. Later the name ‘stunning’ was coined by Park et al. [3].
Mechanism of stunning is based on the poor 131 I accumulation in
residual thyroid tissue on post therapy images when compared to
diagnostic WBI scan. There is an apparent less 131 I uptake than the
amount predicted after high dose 131 I administration for therapy.
Factors that are thought to influence stunning are the following.
a) Type of radiopharmaceutical used
b) Dose effect - Administered activity for diagnostic WBI
scan
c) Amount of residual thyroid remnant
d) Delay between diagnostic WBI scan and treatment,
e) Time between treatment and post therapy WBI scanning,
f) Timing of follow-up studies
g) Effect of thyroid-stimulating hormone and serum iodine
at time of diagnostic testing versus treatment could have an influence
on stunning
Type of RP
Studies have shown that stunning effect can be avoided if 123 I is used instead of 131 I for diagnostic WBI scan purpose. But studies have reported otherwise. Hilditch et al. [4] studied the effect of 131 I therapy on 26 DTC patients using both 123 I and 131 I. 4,000 MBq (108 mCi) was administered as high dose 131 I therapy I3-38 (median 14) days later and uptake in the thyroid bed measured. They concluded that even while using 123I as the diagnostic agent, stunning of the ablation activity still occurs. Both 123 I and 131 I are used for imaging, however 123 I is not available in our country, so we have not tried . Due to the high energy beta and gamma emission from 131 I, an ablative effect is exerted on the neck even with small doses. This especially occurs in patients with minimal residual thyroid tissue. In such instances, thyroid stunning is actually a misnomer or an artifact due to an early therapeutic action effect of ablative 131 I , and not a clinical problem. Once ablated, thyrocytes no longer possess the capacity to accumulate or incorporate 131 I during treatment.
Dose effect
Similarly, dose of administered activity for diagnostic WBI scan is also found to play an important role in inducing stunning. Smaller doses (1-3mCi) are considered better so as not to produce stunning. Higher doses (5-10 mCi) are thought to exaggerate stunning [5,6]. The frequency of stunning was 40%, 67%, and 89% after 3, 5, and 10 mCi (111, 185, and 370 MBq) doses of 131 I, respectively [7]. Cause of stunning is attributed to the cytocidal effects of large amount of thyrocytes, which can imitate a “stunning effect” without any influence on the future effect of 131 I therapy. Alternatively, 131 I may induce a destructive thyroiditis that can cause iodine release from thyroid remnants. Studies have shown that using 131 I at concentrations between 1–3 mCi for diagnosis and administering the therapeutic dose within 24–48 hours prevent the stunning effect
Amount of residual thyroid tissue
Generally, the dosage of 131 I that is used to ablate residual thyroid tissue is in the range of 30 to 50 mCi. Doses less than 35 gray (Gy) to tumor are likely not to respond to 131 I therapy. 300 Gy is needed by thyroid remnant, and 120 Gy by metastatic foci to achieve a good therapeutic response. Although stunning does not distinguish between remnant normal thyroid tissue and metastases, there is no clearcut data on the amount of residual thyroid tissue that can produce stunning. One study showed that presence of LN metastases is important. There was a significant difference in thyroid remnant ablation rate between patients with negative LN metastases (78.1%) and those with LN involvement (59.7 %) (P= 0.01). . On multivariate analysis those who had LN metastases have 2.2 times risk not to enter into successful ablation following the first administered ablative dose [8].
Time delay between scan & therapy
Time delay between diagnostic scan and therapy appears to
be an important factor. It is usually done 5-7 days post therapy.
Cholewinski et al. [9] reported that stunning does not occur if
the ablation therapy is given soon (72 hour) after the diagnostic
activity of 131 I. Sisson et al. [10] showed that 2 days after the
administration of 131 I, the mean fractional concentration of
radioactivity in thyroid tissues after a therapeutic dose is <60%
of the diagnostic dose in most patients, but no correlation of Rx/
Dx with the mCi in the diagnostic dose was seen by them. Being
a radiobiologic phenomenon, there seems to be a correlation
between the absorbed radiation dose and the degree of stunning.
Exact mechanism is unclear. Maximum radiation absorbed dose is
reached within 8-10 weeks. Most of the radiation absorbed dose
occurs within first few days. Ideally the time interval between
pretherapy scan and therapy should not extend too long. Stunning
may result from decreased synthesis of NIS leading to diminished
amounts of iodide transporting protein in pre-irradiated cells. Most
patients show thyroid remnant after attempted total thyroidectomy
requiring iodine ablation. Other factors like timing of follow-up
studies and effect of thyroid-stimulating hormone and serum
iodine at time of diagnostic testing versus treatment could have
a lesser influence on stunning. In all our patients TSH > 30 uIU/
ml (range 30-120 ) and follow WBI scan was strictly performed 6
months after therapy. We recommend that thyroid stunning can be
minimized or completely obliterated by
a) Lowering 131 I diagnostic dosage
b) Shortening the interval between diagnostic WBI scan and
high dose 131 I ablation /therapy
c) Longer interval between 131 I ablation and acquisition of
post therapy WBI scan.
Conclusion
Stunning is radiation dose–dependent, i.e., the higher the radiation-absorbed dose to the target tissue, the greater the stunning effect. Because of concern regarding stunning, many practitioners try to omit the information from a diagnostic WBI scan and proceed directly to 131 I therapy which is not scientifically the right method as dose determination for therapy is much more important than the fear of thyroid stunning. Our study concludes that thyroid stunning is indeed only a visual phenomenon encountered in 17.62% of our DTC patients undergoing high dose 131 I therapy. It however does not affect the therapeutic outcome even in those patients who demonstrated stunning in post therapy scans. Of the various factors studied, it has been found that stunning occurs even while using doses as low as 1.5 mCi 131 I . Other factors that are found to influence the occurrence of stunning are delay of between diagnostic scan and therapy, time between treatment and post therapy WBI scan, and timing of follow-up scans.
Acknowledgements
Acknowledgements: Nil .
Conflicts of Interest
Conflicts of Interest: None.
References
- Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, et al. (2016) Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl J Med 375(7): 614-617.
- Rawson RW, Rall JE, Peacock W (1951) Limitations in the treatment of cancer of the thyroid with radioactive iodine. Trans. Assoc. Am. Physicians 64: 179 -198.
- Park HM (2001) The stunning effect in radioiodine therapy of thyroid cancer. Nucl Med Ann p. 49 -67.
- Hilditch TE, Dempsey MF, Bolster AA, McMenemin RM, Reed NS (2002) Self-stunning in thyroid ablation: evidence from comparative studies of diagnostic 131I and 123I. Eur J Nucl Med Mol Imaging 29(6): 783-788.
- Leger FA, Izembart M, Dagousset F (1998) Decreased uptake of therapeutic doses of iodine-131 after 185-MBq iodine-131 diagnostic imaging for thyroid remnants in differentiated thyroid carcinoma. Eur J Nucl Med 25(3): 242 -246.
- Muratet JP, Giraud P, Daver A, Minier JF, Gamelin E, et al. (1997) Predicting the efficacy of first iodine-131 treatment in differentiated thyroid carcinoma. J Nucl Med 38: 1362 -1368.
- Amdur R, E Mazzaferri (2005) Thyroid Stunning in Essentials of thyroid cancer management Cancer treatment and research. Amdur RJ, Mazzaferri EL (Eds.), Springer, New York, USA p. 55-59.
- Abd El-Kareem M, El-Refaie SH, Zaher A, Abo-Gaba Ml, Abdo S (2012) The Impact of Thyroid Stunning on Radioactive Iodine Ablation Compared to Other Risk Factors. Egyptian J Nucl Med 5(1): 68-78.
- Cholewinski SP, Kenny SY, Klieger PS, O'Mara RE (2000) Absence of Thyroid Stunning After Diagnostic Whole-Body Scanning with 185 MBq 131I. J Nucl Med 41: 1198-1202.
- Sisson JC, Avram AM, Lawson SA, Gauger PG, Doherty GM (2006) The so-called stunning of thyroid tissue. J Nucl Med 47(9): 1406-1412.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...