
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Review Article(ISSN: 2641-1709) 
The Role of Toll-Like Receptors in Allergic Rhinitis Volume 6 - Issue 2
Xindi He and Ying Wang1*
- Department of Rhinology, The First Affiliated Hospital of Zhengzhou University, China
Received: March 22, 2021; Published: April 01, 2021
Corresponding author: Ying Wang, Department of Rhinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
DOI: 10.32474/SJO.2021.06.000232
Abstract
Allergic rhinitis is one of the most common chronic diseases in the world with increasing prevalence, affects the patient’s quality of life and increases the familial and socioeconomic burden. But the exact etiology of allergic rhinitis remains unclear, many studies have demonstrated that Toll-Like Receptors (TLRs) play an important role in the pathogenesis of allergic rhinitis. TLRs, known as Pattern Recognition Receptors (PRRs), are the crucial components of the innate immune system and express either on the cell membrane or in the endolysosomal compartments. TLRs play a crucial role in both innate and adaptive immunity. Each TLR recognizes respective ligand, then recruit a kind of adaptor molecules and initiate downstream signaling events. The signal transduction pathways through TLR are also involved in the pathogenesis of allergic rhinitis. By understanding these mechanisms in allergic rhinitis, we might find a new strategy for improving patient’s quality of life and clinical treatment effect. And the disease symptoms usually cannot be controlled completely with current therapies. The purpose of this review article is to summarize the potential mechanisms of TLRs and the signal transduction pathways through TLR in the pathogenesis of allergic rhinitis and the novel potential therapeutic strategy against allergic rhinitis.
Keywords: Toll-like receptors; allergic rhinitis; innate immunity; adaptive immunity; signal pathway
Introduction
Allergic rhinitis is Immunoglobulin E (IgE) mediated and characterized by accumulation of T-Helper (Th) 2 cytokines and eosinophil inflammation of the nasal mucosa [1]. Its clinical manifestations are pruritus, sneezing, rhinorrhoea, and nasal congestion. As the most common chronic disease, allergic rhinitis still is a severe health problem with increasing prevalence, seriously affects the patient’s quality of life and increases the familial and socioeconomic burden [2,3]. Although the exact etiology of allergic rhinitis remains unclear, it has been demonstrated that genetic, immunological and environmental parameters play important roles in the pathogenesis of allergic rhinitis [4]. Despite advances in the understanding of allergic inflammation mechanisms, and the disease symptoms usually cannot be completely controlled with current therapists we need to explore a new and effective therapy against allergic rhinitis [5]. Concerning the immunopathogenesis, Toll-Like Receptors (TLRs) and TLR signaling pathways both had been demonstrated that they were involved in the pathogenesis of allergic rhinitis. The related research led to this summary of advances in current studies about the relationship between TLRs and TLR signaling pathways and allergic rhinitis. By understanding the potential mechanisms of TLRs and TLR signaling pathways in allergic rhinitis, we might explore a new therapy to improve patient’s quality of life and treatment effect.
Toll-Like Receptors (TLRs)
TLRs belong to the family of Pattern Recognition Receptors (PRRs), which can recognize a wide range of Pathogen-Associated Molecular Patterns (PAMPs)/Damage Associated Molecular Patterns (DAMPs) and participate in the recognition of several pathogens/ allergens and also may participate in the induction of the immune related diseases [6,7]. And TLRs are the crucial components of the innate immune system. TLRs have a similar structure including an extracellular N-terminal domain of approximately 16–28 Leucine- Rich Repeats (LRRs), a transmembrane region and an intracellular C-terminal tail known as Toll/interleukin-1 receptor (TIR). The LRRs domain is responsible for recognition of appropriate ligands, while TIR domain participates in activation of downstream signaling pathway [8,9]. Under normal conditions, commensal bacteria are recognized by TLRs and this recognition is essential for the maintenance of homeostasis and a state of constant controlled inflammation [10]. Different mutations and experimental models, which alter the TLR functions, have demonstrated the significance of TLRs in susceptibility to infection [11,12]. Up to now, 10 members of the TLR family have been recognized in human and 12 (TLR1 to TLR9 and TLR11 to TLR13) in mouse. TLR1, TLR2 , TLR4, TLR5, TLR6, and TLR10 are expressed on the cell membrane and recognize PAMPs of extracellular microbes, whereas TLR3, TLR7, TLR8, and TLR9 are localized in the intracellular endolysosomal compartments and involved in the recognition of nucleic acids [13-15]. Each TLR recognizes distinct PAMPs derived from different pathogens. Upon recognition of respective PAMPs, TLRs recruit a specific set of adaptor molecules that harbor TIR domain and then initiate downstream signaling events that leads to the secretion of Inflammatory Cytokines, type I interferon (IFN), chemokines, and antimicrobial peptides. Then these responses can cause recruitment of neutrophils, activation of macrophages, and induction of IFN-stimulated genes, resulting in direct killing of the infected pathogens [16]. Recognition of self and non-selfantigens, detection of invading pathogens, bridging innate and adaptive immunity, development of antigen-specific immunity are the main functions of TLRs. They also have key roles in cytokine production, apoptosis of infected cells, and release of interferons by viral infected cells [17].
Allergic Rhinitis
Allergic rhinitis is one of the most common allergic disorders globally, affects 10% to 40% of the world’s population and usually persists throughout life. Allergic rhinitis is caused by specific IgEmediated reactions against inhaled allergens driven by Th2 cells and results in nasal mucosal inflammation with tissue influx of eosinophils and basophils [18]. On initial exposure of allergen into the nasal mucosa, the allergen is taken up by antigen-presenting cells (mainly dendritic cells) and processed to a small peptide that binds to specific major histocompatibility complex (MHC) class II molecules. The MHC class II-peptide complex is then expressed on the cell surface, where it is recognized by Th0 receptor and other costimulatory molecules, resulting in differentiation into Th2 CD4+ lymphocytes that produce cytokines like IL-4, IL-5, and IL-13, then promote B cell phenotype switching to produce allergen specific IgE. The allergen-specific IgE binds to its high-affinity receptors (FcεRI) on the surface of mast cells and basophils, causing sensitization of these two cell types. On re-exposure to the specific allergen, crosslinking of the allergen-specific IgE-FcεRI complexes on the mast cell and basophil surfaces triggers secretion of three classes of biologic products: those stored in cytoplasmic granules like histamine, lipid-derived mediators such as leukotrienes, prostaglandins, and platelet-activating factor, and newly synthesized cytokines, chemokines, and growth factors as well as other products. These mediators can cause smooth muscle contraction, increased vascular permeability, mucus secretion and stimulation of sensory nerves, which evoke the symptoms of nasal itching, rhinorrhea, sneezing and congestion. The release of these mediators leads to the early or immediate phase allergic response. These lipid mediators also have chemoattractant properties important for attracting inflammatory cells, particularly Th2 T lymphocytes, eosinophils and basophils into the nasal mucosal tissue, resulting in the late phase allergic response. Late-phase reaction typically develops at 2 to 6 hours after allergen exposure and is characterized by a prolongation of sneezing, rhinorrhea and a predominantly sustained nasal congestion.
There are a variety of mediators and cells which are involved in this late phase. So the symptoms of allergic rhinitis are a result of inhaled allergen-induced inflammation in the nasal mucosa, which is characterized by a Th2-dominated immune response associated with increased levels of serum IgE [19-21]. The main causes of allergy are genetic factors, environmental exposure, and the interaction between both [22]. But immunoregulatory mechanisms are not fully understood, and an imbalance in immune homeostasis predisposes to Th2 immune responses favoring allergic processes [23,24]. In addition, regulatory T cells (Treg cells) [25], Th17 cells [26], group 2 innate lymphoid cells (ILC2s) [27], regulatory B cells and T follicular helper cells [28] may play a role in allergic rhinitis. Allergic rhinitis reduces the quality of life of many patients, impairing sleep quality and cognitive function and causing irritability and fatigue and decreases school and work performance of patients. So appropriate treatment of allergic rhinitis improves symptoms, quality of life, and work and school performance [29]. The therapeutic principle of AR involves a comprehensive approach including environmental control, pharmacotherapy, allergenspecific immunotherapy and patient education. Environmental control should focus on avoidance or reduction of known allergens as well as air pollutants, but it is often hard to achieve. Pharmacotherapy and allergen-specific immunotherapy are the main treatments for allergic rhinitis, but allergic rhinitis is usually not completely curable.
TLRs and TLR signaling pathways in allergic rhinitis
Allergic rhinitis is an IgE-mediated disease which is
predominantly caused by environmental allergen exposure in
genetically predisposed individuals, and the “Th1/Th2” imbalance
has been widely considered to the main pathogenesis of allergic
rhinitis [30,31]. The exact pathogenesis of allergic rhinitis has
remained largely unknown; however, the growing evidence has
shown the relationship between innate immunity and allergic
rhinitis is a causative relationship. And innate immunity plays
an important role in the pathogenesis of inflammation and
inflammatory diseases [32,33]. So innate immunity is a treatment
target for human pro-inflammatory diseases including allergic
diseases. The innate immune system distinguishes pathogens
by special protein receptors. Innate immunity contains several
receptors which have common features regarding ligand recognition, among them, Toll-Like Receptors (TLRs) are the
most known innate immune receptors. TLRs are a conserved
family of transmembrane receptors which recognize the diverse
set of the external and internal molecules and also are expressed
in the different types of white blood cells, such as macrophages,
monocytes, Dendritic cells (DCs), Natural Killer (NK) cells, B and
T lymphocytes, and non-immune cells like fibroblasts, epithelial
cells, and endothelial cells [34,35]. And allergic rhinitis induces
inflammation of the upper respiratory tract, which is associated
with mediators released by several types of hypersensitive immune
cells, including antigen presenting cells, eosinophils, B cells and
mast cells [36].
At present, a large number of studies have shown that TLRs
are widely involved in the pathogenesis of allergic rhinitis. For
example, Renkonen J et al demonstrated that TLR 1-7 and TLR 9-10
proteins are expressed in nasal epithelium, which most probably
reflects to the active innate immunity functions of nasal epithelium
and then causes the occurrence of allergic rhinitis [37]. Cui XY et al
demonstrated the increases in mRNA as well as protein expressions
of TLR2 and TLR4 in patients with Persistent allergic Rhinitis
(PER), and localized the cellular distributions of TLR2 and TLR4 in
the nasal tissues, then thought TLR2 and TLR4 might be one of the
major contributors to the persistence and aggravation of allergic
inflammation in PER [38]. Rich expression of TLRs (TLR3, TLR7,
TLR9) has been found in the nasal epithelial cells of AR patients.
Initial activation of TLRs will trigger the self-protection mechanism,
but excessive activation of TLRs will lead to inflammatory response
due to continuous secretion of pro-inflammatory cytokines and
chemokines [39]. Pattern Recognition Receptors (PRRs) in the form
of Toll-Like Receptors (TLRs) have been immunolocalized within the
sinonasal epithelium. The sinonasal epithelium lies at the interface
between the host and environment and is the primary site of innate
immune interaction. So TLRs participate in the pathogenesis of
allergic rhinitis through affect the innate immunity.In addition
to innate immunity, the involvement of adaptive immunity in the
development of nasal allergic inflammation has been previously
documented. Allergic rhinitis is an IgE mediated specific type I
hypersensitivity reaction, which is induced by the imbalance of Th1
and Th2 immune responses in the body and the nasal mucosal Th2
immune response and is characterized by accumulation of T-helper
(Th) 2 cytokines and eosinophil inflammation of the nasal mucosa
[40]. Therefore, regulating the balance of Th1 and Th2 immune
responses has a preventive and curative effect on allergic rhinitis.
TLRs are pivotal actors in shaping the effective and healthy
adaptive immunity with the development of immune deviation
from the Th2 to Th1 phenotype and maturation of Treg cells [41,42].
TLRs recognize their ligands in homodimeric or heterodimeric
form. All kinds of TLRs are induced alone by their ligands except
TLR2, which is induced via heterodimerization with TLR1 or TLR6
in combination with its ligands. The lipopolysaccharide (LPS)
(TLR4), Single-Stranded Ribonucleic Acid (ssRNA) (TLR7 and
TLR8), Deoxyribonucleic Acid (DNA) (TLR9), Double-Stranded
RNA (dsRNA) (TLR3), lipoproteins (recognized by TLR1, TLR2 and
TLR6), flagellin (TLR5) are some of the ligands that recognized
by TLRs [43,44]. Activation of TLRs has been shown to aggravate
or ameliorate airway reactivity and inflammation. TLR3/ligand
interactions are significantly associated with the deterioration
of allergic rhinitis symptoms via up-regulation of Th1 cytokine,
besides, Contoli et al also reported that Th2 cytokines downregulate
the expression of TLR3 in the bronchial epithelial cell of patients
suffering from allergic rhinitis. However, TLR7 and TLR8/ligand
interactions are associated with the amelioration of allergic
rhinitis symptoms through the suppression of Th2 responses
[9,45]. What’s more, when TLR4 binds to its ligand, it induces
Th0 cells to differentiate into Th2 cells, therefore promotes the
occurrence of allergic rhinitis. And administration of TLR4-short
hairpin RNA (shRNA) alleviates the allergic symptoms of allergic
rhinitis mice by regulating the production of pro inflammatory
mediators [46]. In mice, allergic rhinitis response to house dust
mite might result from TLR2 signaling axis in the nasal mucosa,
whereas in the lung mucosa the allergic asthma response occurs
predominantly via TLR4 signaling axis [47]. And further study
suggests that TLR4 signaling is critically involved in Th2 but not
Th1 sensitization to inhaled antigens [48]. Meanwhile, TLR9 has
been used as a promising target for intervention and treatment of
allergic disease in animal models and clinical trials because of its
ability to mediate a Th1-dominant response to reverse an allergic
phenotype [49]. What’s more, all TLRs except TLR8 are expressed
in the eosinophils, but the expression of TLRs in eosinophils is
relatively low compared to other granulocytes such as neutrophils.
And eosinophils are associated with type 2 immune responses,
including allergic inflammation and helminth parasitic infections.
Studies have shown that eosinophils stimulated with TLR4 ligands
induce upregulation of immune response regulating cytokines [50].
So TLRs participate in the pathogenesis of allergic rhinitis by
affect and regulate the adaptive T cell response and alter the Th1/
Th2 immune balance. What’s more, signal transduction pathways
through TLR are also involved in the pathogenesis of allergic
rhinitis, which is a chronic inflammatory disease of nasal mucosa.
The transmembrane TLR proteins detect the invading pathogens
and binds to the microbial molecules. Following the formation of
TLR and PAMP molecule complex, dimerization of TLRs induces
a cascade of TLR signaling to trigger expression of various genes
like cytokines, chemokines, Mono Histo Compatibility complex
(MHC) and co-stimulatory molecules which are involved in the
host immune function [51]. There are two different TLR signaling
pathways depending on the adaptor molecule : one that requires
myeloid differentiation factor-88 (MyD88) as an adaptor protein or
another that involves the Toll/Interleukin-1 Receptor (TIR) domain
containing adaptor protein inducing IFN-â (TRIF). The TRIFdependent
pathway is considered specific to only few TLRs such
as TLR3 and TLR4, but except TLR3, all TLRs utilize the MyD88-
dependent signaling pathway. The MyD88-dependent signaling
leads to the activation of nuclear factor kappa-B (NF-êB), activating
protein (AP)-1, or interferon regulatory factor (IRF)-1, IRF-5, and IRF-7, while the TRIF-dependent signaling leads to activation of
NF-κB and IRF-3 and its downstream signaling cascade. NF-κB
is the master regulator of all TLR signaling mechanisms and its
activation is the critical event in TLR-mediated activation of the
innate immune response. Although the two types of pathways both
activate NF-κB signaling pathway, the MyD88-dependent pathway
can induce the production of inflammatory cytokines, and the TRIFdependent
pathway is not sufficient to induce cytokine expression
but can induce the production of type I IFN (shown in Figure1)
[17,52-55].
Figure 1: TLRs and their signaling pathways. Ten Toll-like receptors (TLRs) are grouped into extracellular (TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10) and intracellular (TLR3, TLR7, TLR8, and TLR9) subtypes. MyD88 and TRIF are two main pathways in TLR signaling transduction. TLR3 is the only TLR that uses the TRIF-dependent pathway, whereas the other TLRs utilize the MyD88-dependent pathway. Exceptionally, TLR4 can trigger both pathways. These two pathways induce downstream signaling events and leadtoinduction of gene expression (such as proinflammatory cytokines and type I IFNs).
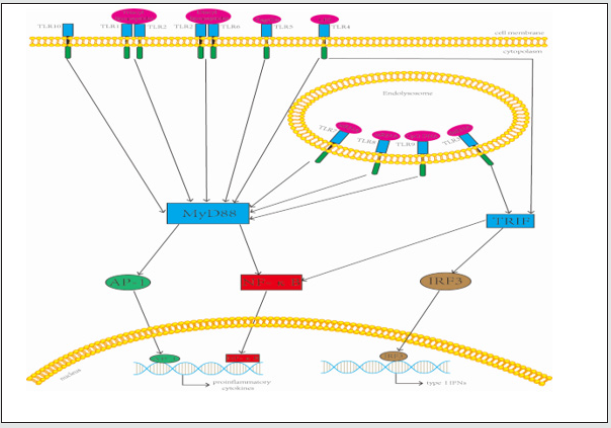
After the NF-κB pathway was activated, the gene transcription of an array of inflammatory cytokines including interleukin-1β (IL-1β), tumor necrosis factor α (TNF-α) is induced, which can reactivate the NF-κB pathway as extracellular stimulation signals. And NF-κB signaling pathway plays an important role in the regulation of cytokine networks of allergic rhinitis [56,57]. NF- κB not only induces the transcription of several cytokines (e.g., TNF-α, IL-1β, and IL- 6) and chemokines (e.g., IL-8, macrophage inflammatory protein-1a, methyl-accepting chemotaxis protein 1 and eotaxin), but also regulates the expression of adhesion molecules (e.g. E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion modecule-1). These cytokines, chemokines and adhesion molecules are more or less related to the occurence of allergic rhinitis. So signal transduction pathways through TLR also are involved in the pathogenesis of allergic rhinitis by synthesis and secretion of pro-inflammatory cytokines and co-stimulatory molecules, which promote inflammatory responses of allergic rhinitis. Figure 1 TLRs and their signaling pathways. Ten Toll-like Receptors (TLRs) are grouped into extracellular (TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10) and intracellular (TLR3, TLR7, TLR8, and TLR9) subtypes. MyD88 and TRIF are two main pathways in TLR signaling transduction. TLR3 is the only TLR that uses the TRIFdependent pathway, whereas the other TLRs utilize the MyD88- dependent pathway. Exceptionally, TLR4 can trigger both pathways. These two pathways induce downstream signaling events and lead to induction of gene expression (such as proinflammatory cytokines and type I IFNs). Therapies of allergic rhinitis mainly include intranasal steroids, antihistamines, leukotriene receptor antagonists and allergen immunotherapy. In these major current therapies of allergic rhinitis, allergen immunotherapy is the only ‘etiologic’ therapy that can change the natural history of allergic disease, but allergen immunotherapy should be continued for a minimum of three years. Pharmacologic therapies can temporarily relieve symptoms and patients need a combination of these drugs; however, once discontinuation of these measures occurs, the immune system often reverts back to the allergic state [58]. Because these therapies have local or systemic side effects, so it is necessery to explore and find new and effective treatments for allergic rhinitis.
TLRs not only participate in the innate immune response but also affect the type and intensity of the acquired immune response, stimulate immune cells to synthesize immune factors and regulate the differentiation of T cells. Activation of TLRs and signal transduction pathway through the TLR both participates in the inflammation and immune response of allergic rhinitis, so TLR is a new target for the treatment of allergic rhinitis. Almost all the TLRs play a role in the development of respiratory allergy, they provide major therapeutic targets to modulate the natural course of allergic disease, but the exact mechanisms need to be further studied. So far, agonists rather than antagonists of TLRs have been widely employed in therapeutic or prophylactic preparations useful for allergic rhinitis/asthma patients [59]. For example, partial TLR4, TLR7, TLR8 and TLR9 agonist significantly reduced sypmptom score and medication score in allergic rhinitis patients. But the therapeutic dose, treatment time and cycle, mode of administration, and many other questions need to be further studied.
Conclusion
Allergic rhinitis is characterized by the inflammation of the nasal mucosa and is one of the most common chronic diseases in the world, with its prevalence rapidly increasing over the past few decades. Treatments of allergic rhinitis mainly include intranasal steroids, antihistamines, leukotriene receptor antagonists and allergen immunotherapy, but the therapeutic effect is still unsatisfactory, so it is necessary to explore novel and effective treatments of allergic rhinitis. Activation of TLRs is not only a part of the innate immune response, but also can affect and regulate the adaptive T cell response and alter the Th1/Th2 immune balance, and mediate related signaling pathway to participate in the pathogenesis of allergic rhinitis. Although TLR is a new target for the treatment of allergic rhinitis, its specific mechanism of action still needs to be further studied. At present, TLR4, TLR7, TLR8 and TLR9 agonist have been used for allergic rhinitis; however, there is a long way to widespread use of such treatment in the treatment of patients with allergic rhinitis.
Acknowledgment
The authors would like to thank Zongping Xia for providing guidance.
Funding
The authors received no specific support or funding for this work.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to research, authorship and/or publication of this article.
References
- Kay AB (2001) Allergy and allergic diseases. First of two parts N Engl J Med 344(1): 30-37.
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, et al. (2008) Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA (2) LEN and AllerGen). Allergy 63(86): 8-160.
- Zhang Y, Zhang L (2019) Increasing Prevalence of Allergic Rhinitis in China. Allergy Asthma Immunol Res 11(2): 156-169.
- Cheng L, Chen J, Fu Q, He S, Li H, et al. (2018) Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis. Allergy Asthma Immunol Res10(4): 300-353.
- Kim DH, Kim SW, Kim SW, Kang JM (2017) Interleukin-37 Relieves Allergic Inflammation in a House Dust Mite Allergic Rhinitis Murine Model. Iran J Allergy Asthma Immunol 16(5): 404-417.
- Kumar H, Kawai T, Akira S (2009) Pathogen recognition in the innate immune response. Biochem J 420(1): 1-16.
- Beutler B (2004) Inferences questions and possibilities in Toll-like receptor signalling. Nature 430(6996): 257-263.
- O Neill LA, Bowie AG (2001) The family of five TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7(5): 353-364.
- Golshiri-Isfahani A, Amizadeh M, Arababadi MK (2018) The roles of toll like receptor 3, 7 and 8 in allergic rhinitis pathogenesis. Allergol Immunopathol (Madr) 46(5): 503-507.
- Kubinak JL, Round JL (2012) Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathog 8(7).
- Parsons KS, Hsu AC, Wark PA (2014) TLR3 and MDA5 signalling though not expression, is impaired in asthmatic epithelial cells in response to rhinovirus infection. Clin Exp Allergy 44(1): 91-101.
- Habibzay M, Saldana JI, Goulding J, Lloyd CM, Hussell T (2012) Altered regulation of Toll-like receptor responses impairs antibacterial immunity in the allergic lung. Mucosal Immunol 5(5): 524-534.
- Takeda K, Akira S (2007) Toll-like receptors. Curr Protoc Immunol.
- Moresco EM, LaVine D, Beutler B (2011) Toll-like receptors. Curr Biol 21(13): 488-493.
- Aryan Z, Rezaei N (2015) Toll-like receptors as targets for allergen immunotherapy. Curr Opin Allergy Clin Immunol 15(6): 568-574.
- Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34(5): 637-650.
- Vidya MK, Kumar VG, Sejian V, Bagath M, Krishnan G, et al. (2018) Toll-like receptors: Significance ligands signaling pathways, and functions in mammals. Int Rev Immunol 37(1): 20-36.
- Wheatley LM, Togias A (2015) Clinical practice. Allergic rhinitis. N Engl J Med 372(5): 456-63.
- Galli SJ, Tsai M, Piliponsky AM (2008) The development of allergic inflammation. Nature 454(7203): 445-54.
- Hansen I, Klimek L, Mösges R, Hörmann K (2004) Mediators of inflammation in the early and the late phase of allergic rhinitis. Curr Opin Allergy Clin Immunol 4(3): 159-63.
- Bernstein DI, Schwartz G, Bernstein JA (2016) Allergic Rhinitis Mechanisms and Treatment. Immunol Allergy Clin North Am 36(2): 261-278.
- Vercelli D (2010) Gene-environment interactions in asthma and allergy the end of the beginning?. Curr Opin Allergy Clin Immunol 10(2): 145-148.
- Soyer OU, Akdis M, Ring J, Behrendt H, Crameri R, et al. (2013) Mechanisms of peripheral tolerance to allergens. Allergy 68(2): 161-170.
- Escribese MM, Gómez-Casado C, Barber D, Diaz-Perales A (2015) Immune Polarization in Allergic Patients Role of the Innate Immune System. J Investig Allergol Clin Immunol 25(4): 251-258.
- Maloy KJ, Powrie F (2001) Regulatory T cells in the control of immune pathology. Nat Immunol 2(9): 816-822.
- Louten J, Boniface K, de Waal Malefyt R (2009) Development and function of TH17 cells in health and disease. J Allergy Clin Immunol 123(5): 1004-1011.
- Doherty TA, Scott D, Walford HH, Khorram N, Lund S, et al. (2014) Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol 133(4): 1203-1205.
- Kim AS, Doherty TA, Karta MR, Das S, Baum R, et al. (2016) Regulatory B cells and T follicular helper cells are reduced in allergic rhinitis. J Allergy Clin Immunol 138(4): 1192-1195.
- Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, et al. (2017) Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines 2016 revision. J Allergy Clin Immunol 140(4): 950-958.
- Eifan AO, Durham SR (2016) Pathogenesis of rhinitis. Clin Exp Allergy 46(9): 1139-1151.
- Amin K (2016) The Role of the T lymphocytes and Remodeling in Asthma. Inflammation 39(4): 1475-1482.
- Melvin TA, Ramanathan M Jr (2012) Role of innate immunity in the pathogenesis of allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg 20(3): 194-198.
- Vijay k (2018) Toll like receptors in immunity and inflammatory diseases Past present and future. Int Immunopharmacol 59: 391-412.
- Faghfouri AH, Zarrin R, Maleki V, Payahoo L, Khajebishak Y (2020) A comprehensive mechanistic review insight into the effects of micronutrients on toll-like receptors functions. Pharmacol Res 152: 104619.
- Lee CC, Avalos AM, Ploegh Hl (2012) Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol 12(3): 168-179.
- Wang Y, Lin L, Zheng C (2012) Downregulation of Orai1 expression in the airway alleviates murine allergic rhinitis. Exp Mol Med 44(3): 177-90.
- Renkonen J, Toppila-Salmi S, Joenväärä S, Mattila P, Parviainen V, et al. (2015) Expression of Toll-like receptors in nasal epithelium in allergic rhinitis. APMIS 123(8): 716-725.
- Cui XY, Chen X, Yu CJ, Yang J, Lin ZP, et al. (2015) Increased expression of toll-like receptors 2 and 4 and related cytokines in persistent allergic rhinitis. Otolaryngol Head Neck Surg 152(2): 233-238.
- Matsushima H, Yamada N, Matsue H, Shimada S (2004) TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol 173(1): 531-541.
- Ciprandi G, Marseglia GL, Castagnoli R, Valsecchi C, Tagliacarne C, et al. (2015) From IgE to clinical trials of allergic rhinitis. Expert Rev Clin Immunol 11(12): 1321-1333.
- Ekman AK, Virtala R, Fransson M, Adner M, Benson M et al. (2012) Systemic up-regulation of TLR4 causes lipopolysaccharide-induced augmentation of nasal cytokine release in allergic rhinitis. Int Arch Allergy Immunol 159(1): 6-14.
- Olivier A, Sainz-Perez A, Dong H, Sparwasser T, Majlessi L, et al. (2011) The adjuvant effect of TLR agonists on CD4(+) effector T cells is under the indirect control of regulatory T cells. Eur J Immunol 41(8) :2303-2313.
- Anwar MA, Shah M, Kim J, Choi S (2019) Recent clinical trends in Toll-like receptor targeting therapeutics. Med Res Rev 39(3): 1053-1090.
- Jahanban-Esfahlan R, Seidi K, Majidinia M, Karimian A, Yousefi B, et al. (2019) Toll-like receptors as novel therapeutic targets for herpes simplex virus infection. Rev Med Virol 29(4): 2048.
- Contoli M, Ito K, Padovani A, Poletti D, Marku B, et al. (2015) Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 70(8):910-920.
- Xu H, Shu H, Zhu J, Song J (2019) Inhibition of TLR4 inhibits allergic responses in murine allergic rhinitis by regulating the NF-κB pathway. Exp Ther Med 18(1): 761-768.
- Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, et al. (2013) Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol 131(2): 549-61.
- Tan AM, Chen HC, Pochard P, Eisenbarth SC, Herrick CA, et al. (2010) TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J Immunol 184(7): 3535-3544.
- Farrokhi S, Abbasirad N, Movahed A, Khazaei HA, Pishjoo M, (2017) TLR9-based immunotherapy for the treatment of allergic diseases. Immunotherapy 9(4): 339-346.
- Yoon J, Um HN, Jang J, Bae YA, Park WJ, et al. (2019) Eosinophil Activation by Toll-Like Receptor 4 Ligands Regulates Macrophage Polarization. Front Cell Dev Biol 20(7): 329.
- Medzhitov R (2001) Toll-like receptors and innate immunity. Nat Rev Immunol 1: 135-145.
- Chen JQ, Szodoray P, Zeher M (2016) Toll-Like Receptor Pathways in Autoimmune Diseases. Clin Rev Allergy Immunol 50(1): 1-17.
- Vandenbon A, Teraguchi S, Akira S, Takeda K, Standley DM (2012) Systems biology approaches to toll-like receptor signaling. Wiley Interdiscip Rev Syst Biol Med 4(5): 497-507.
- De Nardo D (2015) Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine 74(2): 181-189.
- Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11(5): 373-384.
- Wee JH, Zhang YL, Rhee CS, Kim DY (2017) Inhibition of Allergic Response by Intranasal Selective NF-κB Decoy Oligodeoxynucleotides in a Murine Model of Allergic Rhinitis. Allergy Asthma Immunol Res 9(1): 61-69.
- Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2: 17023.
- Rael E (2016) Allergen Immunotherapy. Prim Care 43 :487-94.
- Aryan Z, Holgate ST, Radzioch D, Rezaei N (2014) A new era of targeting the ancient gatekeepers of the immune system: Toll-like agonists in the treatment of allergic rhinitis and asthma. Int Arch Allergy Immunol 164(1): 46-63.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




