
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Case Report(ISSN: 2641-1709) 
Orbital Ameloblastic Carcinoma – Unusual Presentation in A Non-Odontogenic Location Volume 7 - Issue 2
Avinash Arjun Rao Kesari ,Shivakumar Swamy Shivalingappa , Nadezhda Niyarah Alemao*, Lohith G , Indiresh Desai and Veena Ramaswamy
- Department of Radiodiagnosis, HCG Cancer Hospital, India
Received:August 05, 2021; Published:August 16, 2021
Corresponding author: Nadezhda Niyarah Alemao, Department of Radiodiagnosis, HCG Cancer Hospital, Bangalore, India
DOI: 10.32474/SJO.2021.07.000260
Abstract
Ameloblastic carcinoma has been described as an ameloblastoma in which there is histological evidence of carcinoma in a primary or recurrent ameloblastoma. The frequency of the lesion is estimated to be less than 1% of all ameloblastomas occurring in the mandible and maxilla.1 We present a case of a 30-year-old woman who presented with painless swelling of left upper face and recurrent upper respiratory tract infections. She was evaluated with CECT, MRI and PET-CT which showed 3.6 x 3.5 x 3.3 cm mass arising from the inferomedial wall of left orbit with sunburst periosteal reaction and cloudy osteoid matrix which was thought to be an osteosarcoma. She underwent a transorbital and trans nasal endoscopic wide local excision of the lesion which was then diagnosed as ameloblastic carcinoma on histopathology. Imaging features and pathological findings of the tumor along with novel treatment strategies and differential diagnosis are discussed in this case report.
Keywords: MRI; orbital tumor; ameloblastic carcinoma; osteosarcoma; CECT; PET
Abbreviations: 18F-FDG-PET: 18F-fluorodeoxyglucose–Positron Emission Tomography; CECT: Contrast Enhanced Computed Tomography; FLAIR: Fluid Attenuated Inversion Recovery; MRI: Magnetic Resonance Imaging; WHO: World Health Organization
Introduction
Ameloblastic carcinoma has been described as an ameloblastoma in which there is histological evidence of carcinoma either within a primary or a recurrent ameloblastoma. The frequency of the malignancy in an ameloblastoma is estimated to be less than 1% [1]. Ameloblastic carcinoma is not very common with an incidence of around 1-3% of all odontogenic tumors [2-5]. According to the World Health Organisation, ameloblastic carcinoma is classified under the group of odontogenic carcinomas [6,7]. It is a rare malignant tumor that demonstrates histological features of both an ameloblastoma and a carcinoma. The lesion itself can arise de-novo or from malignant transformation of a benign ameloblastoma. This condition has to be differentiated from malignant ameloblastoma which is known to retain benign characteristics in both the primary and metastatic foci in contrary to ameloblastic carcinoma, in which histological features of malignancy are obvious. Ameloblastic carcinoma is often aggressive and can involve lymph nodes and have distant metastasis [8,9]. Therefore, imaging prior to definitive treatment is very important, but detailed literature on imaging findings is very trace [10,11]. Specifically, there is no information about the use of molecular imaging (18F-FDG-PET) in this condition, and in relation to magnetic resonance imaging (MRI) and computerized tomography (CT), very less have been presented [12- 14]. This article highlights a rare presentation of a rare condition presenting at a rare site (orbit).
Case Description
A 30-year-old woman presented with painless swelling of left upper face and excessive watering of eye. She had history of 3 episodes of epistaxis and recurrent upper respiratory tract infections. No history of decreased vision, pain or headache was present. Local examination revealed non-tender proptosis, widening of left interpalpebral region and diplopia of left eye. On diagnostic nasal endoscopy, left nasal cavity showed a 3 x 2 cm submucosal growth in left upper lateral wall with congestion over the swelling. A provisional clinical diagnosis of fibrous dysplasia/ malignancy of left orbit were made and the patient was referred to our institute for further evaluation. The patient was subjected to CECT scan which revealed an ill-defined, lobulated 3.6 x 3.5 x 3.3 cm solid enhancing mass with sunburst periosteal reaction and cloudy osteoid matrix epicentered in inferomedial wall of left orbit.
It was extending to involve the extraconal compartment of left orbit along medial and inferior aspects with involvement of the inferior orbital fissure. The mass also showed extensions to left anterior ethmoid air cells, left nasal cavity, left maxillary infundibulum, left nasolacrimal duct and left malar region (Figures 1 & 2). A radiologic working diagnosis of primary osseous neoplasm was considered with possibility of it being aosteosarcoma.
Figure 1: Contrast CT image soft tissue window (a,b) showing an ill-defined, lobulated mass epic entered in inferomedial wall of left orbit with soft tissue extension to left anterior ethmoid air cells, left nasal cavity, left maxillary sinus, left nasolacrimal duct and extraconal compartment of left orbit (c) shows sunburst periosteal reaction and cloudy osteoid matrix.
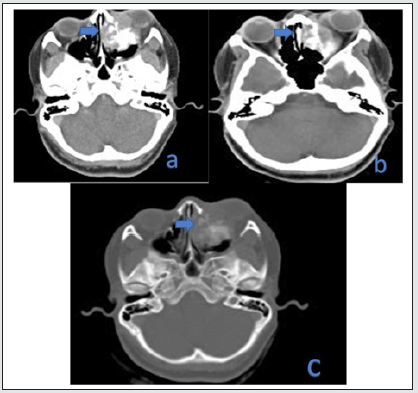
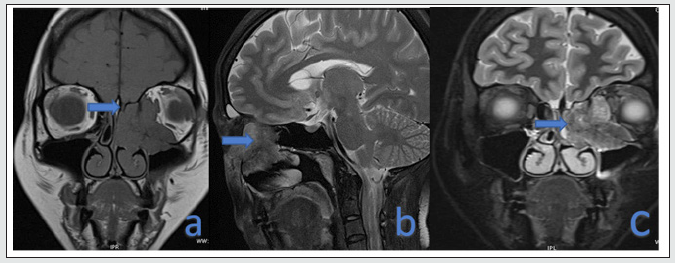
Figure 2: Plain MRI of brain and paranasal sinuses a)T1WI showing intermediate signal intensity within the lesion; b) Sagittal T2W c)Coronal T2W MRI showing hyperintense signal intensity lobulated mass involving the medial, inferior extra canal compartments of left orbit, left maxillary and anterior ethmoid sinuses, left nasolacrimal ducts and left nasal cavity. No intracranial extension was seen.
The patient underwent a preoperative MRI of head and neck which showed a lobulated T2/FLAIR intermediate signal intensity mass involving the medial, inferior extraconal compartments of left orbit, left maxillary and anterior ethmoid sinuses, left nasolacrimal ducts and left nasal cavity with proptosis of left eyeball. No intracranial extension was seen. There was diffusion restriction within the lesion with ADC values ranging between 0.8 x 10-3mm2/s to 1 x 10-3mm2/s (Figure 3). Considering the possibility of an osteosarcoma, staging 18F-FDG-PET-CT was performed which showed increased tracer concentration in the mass with a SUV of about 17.6 (SUV as per Body Weight). No tracer avid regional lymph nodal enlargement or distant metastases were found on PET-CT imaging. The patient then underwent a transorbital and transnasal endoscopic wide local excision of the tumor and complete resection was achieved. Intraoperative frozen section biopsy of the tissue revealed a squamous differentiation. Microscopic examination of the excised specimen revealed odontegenic epithelium in plexiform pattern and in islands in a densely fibrous stroma. The basal cells were columnar and hyperchromatic arranged in a palisaded manner, with centrally located stellate reticulum. Focally the cells exhibited nuclear atypia with vesicular nuclei and inconspicuous nucleoli. Squamous differentiation was noted. Focal areas of necrosis were also seen (Figure 4). The neoplastic cells were seen infiltrating the bone and adjacent adipose tissue. Lymphovascular invasion and perineural invasion was absent. Immunohistochemistry showed immunoreactivity for CK7 and P40. The tumor cells were negative for CD56, Calreinin and S100. Ki-67 proliferative index ranged from 20-25% (Figure 5). Thus, in view of infiltrative pattern and high proliferative index, the diagnosis of ameloblastic carcinoma in a background of ameloblastoma was made. In the follow-up contrast enhanced computed tomography scan 6 months after surgery, the patient was free of recurrence and metastasis (Figure 6).
Figure 3: MRI Brain DWI and ADC maps showing diffusion restriction within the lesion with ADC values ranging between 0.8 x 10-3mm2/s to 1 x 10-3mm2/s.
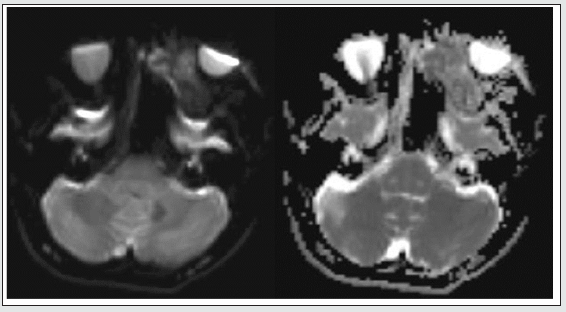
Figure 4: PET-CT showing showed increase tracer concentration in the mass with a SUV of about 17.6 (SUV as per Body Weight).
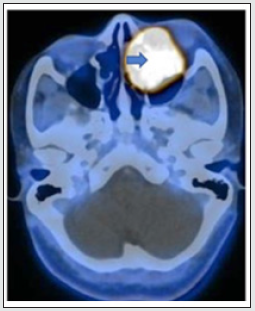
Figure 5: Microscopic examination of tissue revealed odontogenic epithelium in plexiform pattern (blue arrow) and in islands in a densely fibrous stroma. The basal cells were columnar hyperchromatic arranged in a palisaded manner (red arrow). Focally the cells exhibit nuclear atypia with vesicular nuclei and inconspicuous nucleoli. Squamous differentiation and focal areas of necrosis was seen.
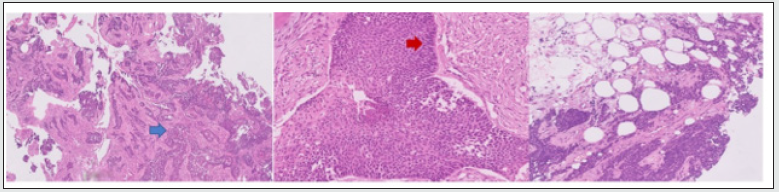
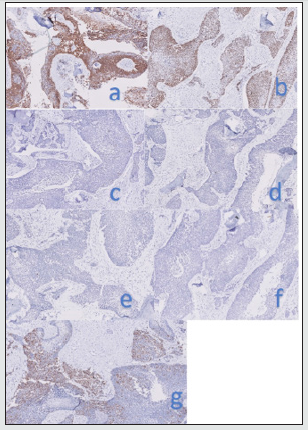
Figure 6: Immunohistochemistry showed immunoreactivity for a) CK7 and b) P40. The tumour cells were negative for c) CK20, d) CD56, e) Calreinin and f) S100. Ki-67 g) proliferative index ranged from 20-25%.
Discussion
Ameloblastic carcinoma was initially described by Shafer, WG et al. and Elzay in 1974 [15,16]. Ameloblastoma and ameloblastic carcinoma can be difficult to differentiate clinically as they share common features. But the latter is more aggressive presenting with rapid growth, cortical destruction, pain and paraesthesia. Clinically, ameloblastic carcinoma has a more aggressive presentation and histologically, it will show features of cytological atypia in addition to the histological features of benign ameloblastoma in the primary tumour [17]. In the 2005 WHO classification of odontogenic tumors, ameloblastic carcinoma was subdivided into primary and secondary type and the latter group further comprised of central and peripheral types. Of the two, the primary type was more common in incidence. But in the present 2017 classification, the subgroups have been removed altogether due to the rarity of the lesion itself [6,18]. Ameloblastic carcinoma is commonly seen with a mean age of 52 years, although it can be seen as early as the second decade [13]. The incidence is equal in both sexes, though controversial male predilection has been described [19]. The most common site of ameloblastic carcinoma is the mandible followed by maxilla [18].
The presentation is usually with pain, swelling with rapid increase in size, trismus and dysphonia. On examination, they can be of varied appearances with indurated ulcers, destruction of underlying bone and mobile teeth [20]. They are usually sessile, solitary, ill-defined lesions that cause tooth loss [21]. Radiographically, these lesions appear as lucent lesions of varying sizes with cortical erosion, soft tissue involvement, local invasion and lymph nodal metastasis [22]. They can also mimic other cystic jaw lesions, appearing as unilocular / multilocular radiolucencies. A sclerotic margin has also been described [21]. The sunburst periosteal reaction and cloudy osteoid matrix noted in our case has not been described in literature. Also, due to increased tracer concentration in the mass on 18F-FDG-PET-CT, this investigation can further be explored to delineate malignant transformation early [23].
On histopathological evaluation, these lesions show features of cytological atypia such as necrosis, increased mitotic index, vascular invasion, neural invasion, squamous metaplasia and keratin pearl formation along with other features of benign ameloblastoma such as follicular pattern with peripheral palisading ameloblasts [24]. Wide local excision is the mainstay of treatment of these lesions with close post-operative follow-up in view of high incidence of local recurrence [24]. Most of the reported cases of ameloblastic carcinoma have been treated with aggressive surgery with margin of 2-3 cm, while Gamma Knife stereotactic radiosurgery has also been described recently [25-27]. Prognosis is said to be no better than a malignant ameloblastoma, hence early diagnosis of the lesion is helpful [28]. Prominent differential diagnosis of ameloblastic carcinoma include squamous cell carcinoma, acanthomatous ameloblastoma, kerato-ameloblastoma and primary intra-alveolar epidermoid carcinoma. To our knowledge, this is the first reported case of an ameloblastic carcinoma in the orbit. There have been few cases of maxillary amelobastomas with secondary involvement of the orbit [29]. Most of the reported cases of ameloblastic carcinoma have been in maxilla or mandible. Our case also was completely solid without cystic components and had sunburst periosteal reaction which gave rise to the diagnostic dilemma.
Conclusion
The presenting case was one among the very few cases reported in literature, with primary involvement of the orbit having never been described before. The lesion itself can present with atypical features as in our case and hence can be confused with other sitespecific lesions. 18F-FDG-PET-CT imaging can further be explored to delineate malignant transformation. Since ameloblastic carcinoma exhibits features of both ameloblastoma and a carcinoma, aggressive treatment strategies are to be employed at early stages for good prognosis.
Key learning points:
a) Identification of early malignant transformation in ameloblastoma can play a pivotal role in patient management.
b) A remote possibility of ameloblastic carcinoma should be kept in mind while evaluating orbital lesions.
c) Mostly ameloblastic carcinomas mimic other cystic jaw lesions, appearing as unilocular or multilocular radiolucencies; however, a sclerotic margin, sunburst periosteal reaction and cloudy osteoid matrix can be visualized as in our case.
Declaration
It is certified that the authors have obtained all appropriate patient consent forms in which the patient has given consent for the images and other clinical information to be reported in the journal. The patient understands that their names and initials will not be published, and due efforts will be made to conceal their identity.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
None
References
- Prashad KV, Ramesh V, Balamurali PD, Premalatha B (2011) Ameloblastic Carcinoma. A case report highlighting its variations in histology. J Int Oral Health 3(6): 37–42.
- Ladeinde AL, Ajayi OF, Ogunlewe MO, Adeyemo WL, Arotiba GT, et al. (2005) Odontogenic tumors: A review of 319 cases in a Nigerian teaching hospital. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 99: 191-195.
- Buchner A, Merrell PW (2006) Relative frequency of central odontogenic tumors: A study of 1,088 cases from Northern California and comparison to studies from other parts of the world. J OralMaxillofac Surg 64: 1343-1352.
- Jing W, Xuan M, Lin Y, Wu L, Liu L, et al. (2007) Odontogenic tumors: A retrospective study of 1642 cases in a Chinese population. Int J Oral Maxillofac Surg 36: 20-25.
- Taghavi N, Mehrdad L, Rajabi M, Akbarzadeh A (2010) A 10-yearretrospective study on malignant jaw tumors in Iran. J Craniofac Surg 21: 1816-1819.
- Wright J, Soluk Tekkeşin M (2017) Odontogenic Tumors. Where are we in 2017?. Journal of Istanbul University Faculty of Dentistry 51(3 Supp 1): 10-30..
- Wright J, Vered M (2017) Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Odontogenic and Maxillofacial Bone Tumors. Head and Neck Pathology 11(1): 68-77.
- Jindal C, Palaskar S, Kaur H, Shankari M (2010) Low-grade spindlecell ameloblastic carcinoma: report of an unusual case with immunohistochemical findings and review of the literature. Curr Oncol 17: 52-57.
- Karakida K, Aoki T, Sakamoto H, Takahashi M, Akamatsu T, et al. (2010) Ameloblastic carcinoma, secondary type: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110: e33-37.
- Kruse AL, Zwahlen RA, Grätz KW (2009) New classification of maxillary ameloblastic carcinoma based on an evidence-based literature review over the last 60 years. Head Neck Oncol 1: 31.
- Lucca M, d’Innocenzo R, Kraus JA, Gagari E, Hall J, et al. (2010) Ameloblastic carcinoma of the maxilla: a report of 2 cases. J Oral Maxillofac Surg 68: 2564-2569.
- Yoon HJ, Hong SP, Lee JI, Lee SS, Hong SD (2009) Ameloblastic carcinoma: an analysis of 6 cases with review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108: 904-913.
- Akrish S, Buchner A, Shoshani Y, Vered M, Dayan D (2007) Ameloblastic carcinoma: report of a new case, literature review, and comparison to ameloblastoma. J Oral Maxillofac Surg 65: 777-783.
- Devenney-Cakir B, Dunfee B, Subramaniam R, Sundararajan D, Mehra P, Spiegel J, et al. (2010) Ameloblastic carcinoma of the mandible with metastasis to the skull and lung: advanced imaging appearance including computed tomography, magnetic resonance imaging and positron emission tomography computed tomography. Dentomaxillofac Radiol 39: 449-453.
- Elzay R (1982) Primary intraosseous carcinoma of the jaws: Review and update of odontogenic carcinomas. Oral Surgery, Oral Medicine, Oral Pathology 54(3): 299-303.
- Shafer WG, Hine MK, Levy BM (1974) A Textbook of Oral Pathology, ed 3, Philadelphia, WB Saunders Company, USA pp. 254.
- Yoon H, Jo B, Shin W, Cho Y, Lee J, et al. (2011) Comparative immunohistochemical study of ameloblastoma and ameloblastic carcinoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 112(6): 767-776.
- Deng L, Wang R, Yang M, Li W, Zou L (2019) Ameloblastic carcinoma: Clinicopathological analysis of 18 cases and a systematic review. Head & Neck 41(12): 4191-4198.
- Li J, Du H, Li P, Zhang J, Tian W, et al. (2014) Ameloblastic carcinoma: An analysis of 12 cases with a review of the literature. Oncology Letters 8(2): 914-920.
- Kar IB, Subramanyam RV, Mishra N, Singh AK (2014) Ameloblastic carcinoma: Report of two cases. Ann Maxillofac Surg 4(1): 70–77.
- Cho B, Jung Y, Hwang J (2020) Ameloblastic carcinoma of the mandible: A case report. Imaging Science in Dentistry 50(4): 359.
- Kodati S (2016) Ameloblastic Carcinoma: A Report of Three Cases. Journal of Clinical and Diagnostic Research 10(10): ZD23-ZD25.
- Ohira M, Kawahara K, Oriyama T, Oguri T, Shibata A, et al. (2020) Secondary ameloblastic carcinoma in the midline of the mandible: A case report and literature review. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology 32(2): 109-113.
- Soyele O, Adebiyi K, Adesina O, Ladeji A, Aborisade A, et al. (2018) Ameloblastic carcinoma: a clinicopathologic analysis of cases seen in a Nigerian Teaching Hospital and review of literature. Pan African Medical Journal 31: 208.
- Benlyazid A, Lacroix-Triki M, Aziza R, Gomez-Brouchet A, Guichard M, et al. (2007) Ameloblastic carcinoma of the maxilla: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104: 17–24.
- Hall JM, Weathers DR, Unni KK (2007) Ameloblastic carcinoma: an analysis of 14 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:799–807.
- Perera E, Lindquist C, Hughes C, Thomas S (2013) The use of Gamma Knife stereotactic radiosurgery in the treatment of ameloblastic carcinoma. International Journal of Oral and Maxillofacial Surgery 42(8): 934-938.
- Ram H, Mohammad S, Husain N, Gupta P (2010) Ameloblastic Carcinoma. Journal of Maxillofacial and Oral Surgery 9(4): 415-419.
- Milman T, Lee V, Li Volsi V (2016) Maxillary Ameloblastoma with Orbital Involvement. Ophthalmic Plastic and Reconstructive Surgery 32(6): 441-446.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




