Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Research Article(ISSN: 2641-1709) 
Gustatory Dysfunction in Covid 19 Patients: A Cross Sectional Study Volume 5 - Issue 4
Abhishek M1*, Bandiahanapalya Narasappa Yogesh1, Borlingegowda Viswanatha2, Harshavardhan Annigeri1 and Sahana Raju3
- 1Senior Resident, Department of Otorhinolaryngology, Bangalore Medical College and Research Institute, India
- 2Professor and Head, Department of Otorhinolaryngology, Bangalore Medical College and Research Institute, India
- 3Junior Resident, Department of Otorhinolaryngology, Bangalore Medical College and Research Institute, India
Received: December 18, 2020 Published: January 06, 2021
Corresponding author: Abhishek M, Senior Resident, Department of Otorhinolaryngology, Bangalore Medical College and Research Institute, India
DOI: 10.32474/SJO.2021.05.000221
Abstract
Background: Loss of smell and taste are considered potential discriminatory symptoms indicating triaging for coronavirus disease 2019 (COVID-19) and early case identification. However, the estimated prevalence essential to guide public health policy varies in published literature.
Methods: The prospective study evaluated 100 individuals with a COVID-19 infection, as confirmed by Reverse transcriptase PCR laboratory testing. Olfactory and gustatory testing were carried out by an examiner utilizing stringent safety standards and wearing full personal protective equipment.
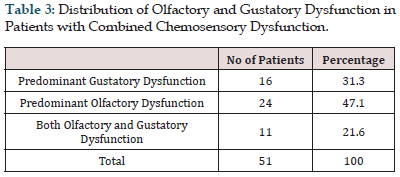
Results: Among the 100 patients included in this study, 54% were male and 46% were female with a mean age of 38.82±12.21 years (18-72). Among the 100 patients included in this study, 13 patients had isolated taste dysfunction, 16 patients had isolated olfactory dysfunction, 51 patients had combined dysfunction, and 20 patients had neither taste nor olfactory dysfunction. Among the 51 patients who had combined olfactory and gustatory dysfunction, 31.3 % of them had predominant gustatory dysfunction, 47.1 % predominant olfactory dysfunction and 21.6% of the patients had both olfactory and gustatory dysfunction equally.
Conclusions: Olfactory and gustatory dysfunctions are strongly associated with SARS-CoV-2 infection. Olfactory with or without gustatory dysfunctions is potentially a reliable indicator of latent COVID-19.
Keywords:COVID-19; gustatory dysfunction; taste; SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus, first presented in December 2019 in Wuhan, China, and has quickly spread all around the world [1,2]. It was declared by the World Health Organization as a pandemic and global health emergency on March 11, 2020 [3]. Across the globe, the SARSCoV- 2 virus has infected millions of individuals and killed hundreds of thousands. In January 2020, angiotensin-converting enzyme 2 (ACE-2) was identified as the functional receptor for SARS-CoV-2, present in multiple human organs including the central nervous system. The common symptoms of COVID-19 are general malaise, fever, cough, and shortness of breath. Other symptoms include muscle and joint pain, sore throat, headache, nausea, or vomiting, diarrhoea, and some nasal symptoms, especially smell and taste dysfunction. Like other upper airway viral infections (URTI), such as common cold or flu, the loss of smell is a frequent symptom in COVID-19 patients. However, a sudden, severe, and isolated loss of smell and/or taste may also be present in COVID-19 patients who are otherwise asymptomatic [4]. The nasal and oral cavities are a common portal of entry, and it is known that the viral organism gets adapted and multiplies therein before being propagated to the lower respiratory tract. In the last few months, researchers have been focusing mainly on the analysis of olfactory disorders and have often overlooked taste dysfunctions, mainly for the following two reasons:
a) The olfactory pathway could be a way of access to the central nervous system for the coronavirus [5]. Thus, the olfactory dysfunctions could represent a sign of neuro invasion, while ageusia and hypogeusia would not have any possible interesting pathogenetic implication.
b) The gustatory disturbances have generally been classified as a result of the retro nasal olfactory loss [6]. The aim of this study is to evaluate the prevalence of gustatory disturbance i.e., ageusia/hypogeusia among the COVID 19 patients.
Methods
This prospective study was undertaken in Bangalore medical college and research center, a designated COVID hospital. The study evaluated a consecutive series of patients between September 2020 and October 2020. All the patients enrolled in the study were diagnosed with COVID-19. 100 hospitalized patients were included in this cross sectional study satisfying the inclusion and exclusion criteria.
Inclusion criteria
a) Age> 18 years.
b) Laboratory-confirmed COVID-19 infection (reverse transcription−polymerase chain reaction, RT-PCR).
c) Patients clinically stable.
) Patients giving consent to participate in the study.
Exclusion criteria
a) Patients without a laboratory-confirmed diagnosis of COVID-19 infection.
b) Patients in the intensive care unit.
c) Patients with olfactory or gustatory dysfunctions before the epidemic due to congenital anosmia, the side effects of drugs (chemotherapy), previous surgery or radiotherapy in the oral and nasal cavities, head injury, sinonasal diseases, allergic rhinitis.
d) Patients with systemic diseases (iron deficiency, autoimmune diseases).
e) Patients with neurodegenerative disorders (Parkinson’s disease, disease Alzheimer’s disease, dementia).
f) Patients with major depression.
Gustatory testing
Gustatory testing was carried out by the examiners utilizing stringent safety standards and wearing full personal protective equipment. Gustatory testing involved the identification of three solutions in the water representing different types of taste: sweet, salty, and sour.
a) Sweet-tasting solution was 50% sucrose.
b) Saltiness was tested using 0.9% saline solution.
c) Sour taste, 4.2% vinegar was given.
The testing hours were during the morning to prevent any confounding effects from circadian alterations. The subjects had not eaten or drunk anything within the hour preceding the test. Each participant rinsed his or her mouth with distilled water first. Swab sticks were dipped into the previously prepared solutions and applied to the anterior two-thirds of the lingual dorsum. The identification of the taste was recorded for each taste and for each subject. The participants used distilled water to flush out their mouths completely between the application of the next solution.
Olfactory testing
Olfaction was tested using common household items like soap, coffee, cinnamon, and lemon. The odorants were presented one at a time, in identical and opaque containers covered by gauze.
Results
Among the 100 patients included in this study, 54% were male and 46% were female with a mean age of 38.82±12.21 years (18-72) (Figure 1 & Table 1). Among the 100 patients included in this study, 13 patients had isolated taste dysfunction, 16 patients had isolated olfactory dysfunction, 51 patients had combined dysfunction, and 20 patients had neither taste nor olfactory dysfunction (Figure 2 & Table 2). Among the 51 patients who had combined olfactory and gustatory dysfunction, 31.3 % of them had predominant gustatory dysfunction, 47.1 % predominant olfactory dysfunction and 21.6% of the patients had both olfactory and gustatory dysfunction equally (Figure 3 & Table 3).
Table 1: Sex Distribution.
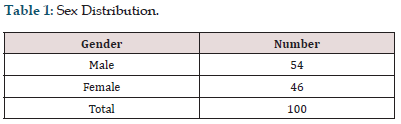
Figure 1: Sex Distribution.
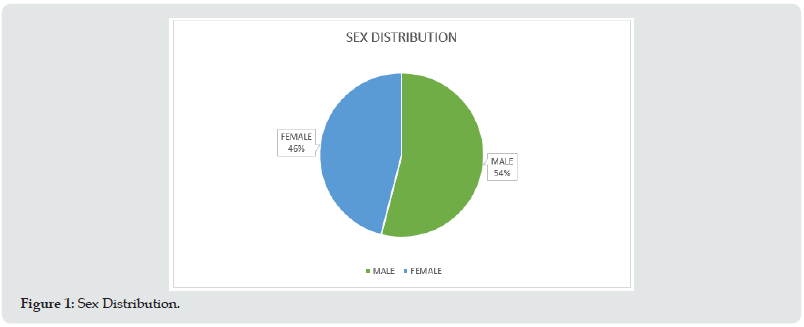
Figure 2: Olfactory and Taste Dysfunction.
Figure 3: Distribution of Olfactory and Gustatory Dysfunction in Patients with Combined Chemosensory Dysfunction.
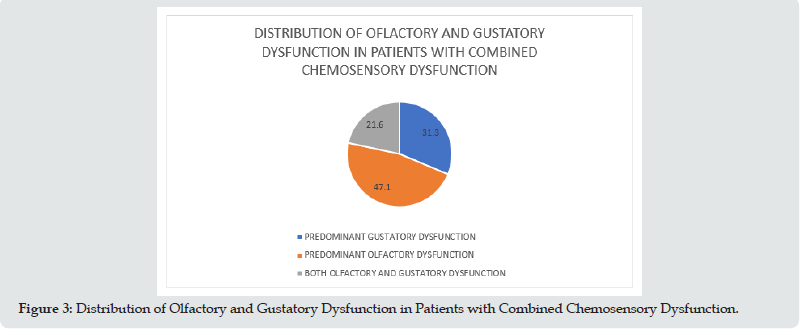
Table 3: Distribution of Olfactory and Gustatory Dysfunction in Patients with Combined Chemosensory Dysfunction
Discussion
From the start of the COVID-19 pandemic, it has been proposed that acute onset olfactory +/- gustatory dysfunction may represent an early marker of probable infection [7]. Olfactory and gustatory dysfunction may occur even in the absence of other symptoms suggestive of COVID-19. Although it is known that coronaviruses cause the common cold, many individuals presenting to ENT witha common cold accompanied by olfactory or gustatory dysfunction are given a diagnosis of post-viral anosmia [8]. Xu et al. [9] evaluated the expression of ACE2 receptor in the oral cavity by examining RNA transcripts. The lingual expression of ACE2 was higher than in the cheek or gingiva. This differential expression may partly account for the gustatory loss seen in SARSCoV-2 infection. Our results indicate that gustatory dysfunction occurred in 64%of confirmed cases of COVID-19. It is rare to encounter dysgeusia as the sole presenting complaint, as it generally occurs in association with anosmia, as it does in COVID-19 cases. Klopfenstein et al. [10] report that anosmia and dysgeusia co-occurred 85% of the time and where a nasal discharge was present, in over 50% of cases. The results of the present study indicate an occurrence of anosmia alone in 16%. Dysgeusia without anosmia was only present in 13% of cases, whilst co-occurrence of the two symptoms were present in 51%. These results support the notion that mechanical blockage of the nose is not the cause of anosmia in COVID 19 cases.
Anecdotal evidence favors considering hyposmia or hypogeusia as early indicators of SARS-CoV-2 infection before the clinical syndrome fully develops. At present, nonetheless, there is a lack of clarity regarding how far such symptoms are a risk factor for hospital admission, or result of the virus or are just due to observer bias (i.e., seeing what it is expected will be seen). Establishing the temporal relationship between COVID-19 and hyposmia/ hypogeusia is essential if these symptoms are of value as markers of otherwise asymptomatic disease and hence to identify contagious individuals. The cases enrolled in this study were hospital patients at the time of assessment but data regarding their clinical outcome was not available to the researchers at the time of writing. The study needs to be extended to assess the duration of symptoms and their resolution. The study was undertaken when COVID-19 was a pandemic and the use of advanced imaging and diagnostic modalities, such as endoscopy, paranasal sinus tomography and/or magnetic resonance imaging, were contraindicated due to the risk of cross-infection by the virus.
Conclusion
Healthcare workers should be warned that in patients presenting with the isolated sudden onset of ageusia, ongoing coronavirus infection should be suspected. For these reasons, in our opinion it is essential that gustatory dysfunctions, like olfactory disorders, are included in the COVID-19 guidelines as they are frequent, highly specific, and smell-independent symptoms of SARS-CoV-2 infection.
References
- Wu D, Wu T, Liu Q, Yang Z (2020) The SARS-CoV-2 outbreak: what we know. Int J Infect Dis 94: 44-48.
- Guan WJ, Ni ZY, Hu Y (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708- 1720.
- Mahase E (2020) Covid-19: WHO declares pandemic because of ‘‘alarming levels’’ of spread, severity, and inaction. BMJ 368: m1036.
- Mullol J, Alobid I, Mariño-Sánchez F, Izquierdo-Domínguez A, Marin C, et al. (2020) The Loss of Smell and Taste in the COVID-19 Outbreak: A Tale of Many Countries. Current Allergy and Asthma Reports 20(61).
- Vavougios GD (2020) Potentially irreversible olfactory and gustatory impairments in COVID-19. Indolent versus fulminant SARS-CoV-2 neuroinfection. Brain Behav Immun 87:107–108.
- Whitcroft KL, Hummel T (2020) Olfactory dysfunction in COVID-19. Diagnosis and management. JAMA 323: 2512–2514.
- Bagheri SHR, Asghari AM, Farhadi M (2020) Coincidence ofCOVID-19 epidemic and olfactory dysfunction outbreak. J Infect 34: 62.
- Gilani S, Roditi R, Naraghi M (2020) COVID-19 and anosmiain Tehran, Iran. Med Hypotheses 141: 109757.
- Xu H, Zhong L, Deng J (2020) High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 12(1).
- Klopfenstein T, Kadiane-Oussou NJ, Toko L (2020) Features of anosmia in COVID-19. Medecine et Maladies Infectiousness 50(5).

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...