Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Research Article(ISSN: 2641-1709) 
Evaluation of Serum Vitamin D Level in Babies Followed with the Diagnosis of Laryngomalacia Volume 9 - Issue 3
Serdar Ferit Toprak1, Muhammed Aryal2 and Serkan Dedeoglu3*
- 1Department of Audiology, Artuklu University, Mardin, Turkey
- 2Department of Otorhinolaryngology, Dicle University, Diyarbakır, Turkey
- 3Department of Otorhinolaryngology, University of Health Sciences Gazi Yasargil Training and Research Hospital, Turkey
Received: November 11, 2022; Published: November 25, 2022
Corresponding author: Serkan Dedeoglu, Department of Otorhinolaryngology, Diyarbakır, University of Health Sciences Gazi Yasargil Training and Research Hospital, Turkey
DOI: 10.32474/SJO.2022.09.000313
Abstract
Objectives: The relationship between vitamin D and laryngomalacia is not fully known. Our study aimed to investigate whether there is any relationship between laryngomalacia and vitamin D.
Materials and Methods: Thirty-two patients under one year of age with a diagnosis of laryngomalacia without any other disease were included in our study. Anamnesis, physical examination, and examination with a flexible endoscope were performed. Serum levels of calcium, parathormone (PTH), and vitamin D were analyzed as laboratory tests and compared between the two groups.
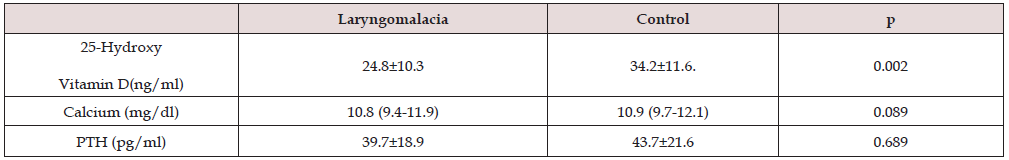
Results: There was a significant decrease in serum vitamin D levels compared with the control group infants (p = 0.002). There was no significant difference in serum calcium and parathormone levels (Calcium p = 0.089, PTH p = 0.689).
Conclusions: In our study, Vitamin D levels of infants in the control group were found to be higher than those of infants with laryngomalacia. However, there was no difference in parathormone and calcium serum levels. We suggest that vitamin D deficiency may cause the etiology of laryngomalacia, which has not been entirely determined.
Keywords: Stridor; Parathormone; Calcium; Laryngomalacia
Abbreviations: PTH: Parathormone; 25-OH D: 25-Hydroxy Vitamin D; SD: Standard Deviation; SPSS: Statistical Package for Social Sciences
Introduction
Laryngomalacia can be defined as the inward collapse of the supraglottic region during respiratory intake [1]. It is the most common congenital laryngeal anomaly in infants, causing 45% to 75% of stridor symptoms in infants under one year of age [2]. The most important symptom is a loud breathing problem that increases with breathing. Difficulty breathing is worse with crying, breastfeeding, and lying down. Symptoms begin after birth and usually disappear after the age of two years [1,3]. Although laryngomalacia is seen as a disease with mild symptoms in the first 24 months of life in children followed up with a diagnosis of laryngomalacia, respiratory distress requiring surgery may occur in a very small number of children [2]. The diagnosis is made by observation of supraglottic collapse on flexible laryngoscopic examination [4]. Although surgery is not considered in many babies with laryngomalacia, this intervention may be necessary if the baby is not developing and has a severe respiratory failure [4,5]. The exact cause and pathophysiology of laryngomalacia are still unknown.
Different theories are considered in the etiology of laryngomalacia. Incomplete cartilage development, muscular and nervous system disorders, reflux are the most common causes [5-7]. Since these theories cannot fully explain the reason and pathophysiology of laryngomalacia, studies in this field continue. There is much research on vitamin D. Although vitamin D is an important cellular vitamin, its deficiency can cause many diseases [8-10]. There are articles in the literature that try to find the effect of vitamin D [11,12]. It was observed that most of these studies were on extremity diseases, and there were very few studies on vitamin D related to the larynx and trachea. Although vitamin D deficiency is known to be associated with congenital stridor [13,14]. an etiological link of vitamin D with laryngomalacia has not yet been found. Another issue is that publications have investigated the relationship between chondromalacia and vitamin D serum levels [15,16]. By analyzing these studies, we thought it was essential to investigate the link between vitamin D and laryngomalacia. In our study, we aimed to find the relationship between the amount of vitamin D in the blood and patients with laryngomalacia. This study aimed to examine whether vitamin D serum levels are low in infants with laryngomalacia.
Materials and Methods
This study was conducted by the principles of Good Clinical Practice and the Declaration of Helsinki. This case-controlled, non-randomized study was performed on patients consulted or admitted to the otorhinolaryngology outpatient clinic from pediatric outpatient clinics. The study group included 32 infants (21 boys, 11 girls) who were less than one year old, born at a typical birth week, had no congenital disease and were diagnosed with laryngomalacia by endoscopy. Other genetic respiratory tract diseases (such as hemangioma in the vocal cord region, tracheal obstruction), wheezing after birth (history of croup and mechanical ventilator dependence), infectious or nervous system diseases, and babies thought to have genetic diseases were determined as exclusion criteria. As a control group, 38 healthy infants (28 boys, 10 girls) younger than one year of age without signs of laryngomalacia were included. Detailed anamnesis, systemic physical examination, flexible laryngoscopic examination, and laboratory tests (25-Hydroxy vitamin D (25-OH D), calcium (Ca), and parathormone (PTH)) were performed. In the detailed anamnesis, it was questioned whether wheezing was present with breathing and whether respiratory distress increased with breastfeeding or lying down.
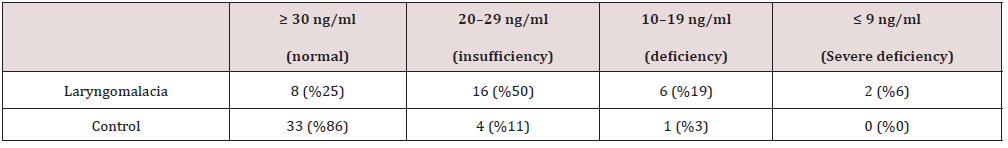
On physical examination, the presence of suprasternal retraction was examined. A laryngoscopic exam was performed with a flexible laryngoscope in the otorhinolaryngology outpatient clinic. The babies were placed on their mothers’ laps, and the laryngeal region was observed by entering the nostrils with the endoscope and advancing the nasal cavity nasopharynx oropharynx. This examination revealed laryngomalacia when the supraglottic collapse was observed during inspiration. None of the infants required operation. All sick and healthy infants were given 400 units of vitamin D daily by mouth from birth within the scope of the vitamin D supplementation and administration program of the Ministry of Health. Vitamin D serum levels were measured after informed consent was obtained from the parents of patients with laryngomalacia and healthy individuals selected as the control group. Ca, PTH, and 25-OH D levels were measured in the serum obtained by centrifuging the blood. Patients were divided into standard (≥ 30 ng/ml), deficiency (29-20 ng/ml), deficiency (19-10 ng/ml), and severe deficiency groups according to vitamin D levels (≤ 9 ng/ml and below) [17,18]. The ethics committee approved this study of Dicle University Faculty of Medicine at its meeting dated 10.11.2021 with ethics committee number 445.
Statistical Analysis
IBM SPSS Statistics 20 (Statistical Package for Social Sciences v.21, IBM, Chicago, IL) program was used to calculate statistical data. Data were expressed as mean and standard deviation (SD). Student’s t-test was used to compare the means between cases and controls, with a p-value of < .05 considered statistically significant. 25-Hydroxy vitamin D, calcium, and parathormone were analyzed using the Kolmogorov-Smirnov test. Of the 70 infants in our study, 49 were boys, and 21 were girls. The mean age was 4.83 ± 1.23 months. It was found that there was no significant difference between healthy infants and those diagnosed with laryngomalacia. Table 2 shows a substantial decrease in serum 25-hydroxy vitamin D levels compared to the control group infants (p = 0.002). There was no significant difference in serum calcium and parathormone levels (p = 0.089 for calcium and p = 0.689 for PTH).
Discussion
Laryngomalacia is an anomaly of the larynx that occurs from birth. It is the most common cause of stridor in the neonatal period. It has an appearance characterized by supraglottic collapse. The etiopathogenesis is still unexplained [1-3]. In the literature, very few publications investigate vitamin D and laryngomalacia in infants. In our study, the relationship between vitamin D and children with laryngomalacia was tried to be found. Vitamin D levels of infants in the control group were found to be higher than those of infants with laryngomalacia. Many research articles have also shown that serum vitamin D levels of healthy infants are higher than those of infants with laryngomalacia. [3,19]. In our study, higher serum vitamin D levels were found in the control group infants compared to the infants followed up with a diagnosis of laryngomalacia.
There was no significant difference in serum calcium and PTH levels. Typically, PTH serum levels should be elevated in infants with laryngomalacia who had low serum vitamin D levels, but no elevation was observed. The function of vitamin D in the body is to increase the absorption of Ca from the intestines and to prevent the excretion of Ca through the urine in renal function, and PTH contributes to the increase in serum vitamin D levels.PTH receptors are involved in the maturation of cartilage tissues [20, 21]. Numerous articles investigate the relationship between vitamin D and chondromalacia [15,16,22]. Osteoarthritis and vitamin D deficiency have been associated with disease progression [23].
Immature chondrocytes in the larynx may be thought to cause laryngomalacia. Studies in the literature suggest that vitamin D influences brain and nerve formation in the intrauterine period [24,25]. Because of this data, vitamin D deficiency may be considered a cause of laryngomalacia. However, normal PTH serum levels, instead of PTH serum levels expected to increase in response to vitamin D deficiency, raise questions such as whether there is any problem with PTH receptors and whether these receptors have not completed their proliferation or maturation. Therefore, further studies with PTH may provide insight into the cause of laryngomalacia. During pregnancy, vitamin D of the fetus comes from the mother through the placenta. A mother’s vitamin D deficiency may cause the baby’s vitamin D deficiency. It may be necessary to examine vitamin D serum levels in mothers. In future studies, maternal serum vitamin D levels may provide valuable information for the etiology of laryngomalacia.
Conclusion
In this study, we suggest that vitamin D deficiency may cause the etiology of laryngomalacia, which has not been entirely determined. In addition, investigating serum vitamin D deficiency in pregnant women may help explain the etiopathogenesis of laryngomalacia with low vitamin D deficiency in newborn babies.
References
- Ayari S, Aubertin G, Girschig H, Van Den Abbeele T, Mondain M (2012) Pathophysiology and diagnostic approach to laryngomalacia in infants. European annals of otorhinolaryngology, head and neck diseases 129(5): 257-263.
- Landry AM, Thompson DM (2012) Laryngomalacia: disease presentation, spectrum, and management. International journal of pediatrics 2012: 753526.
- Hassan MM, Emam AM, Mahmoud AM, Awad AH, Rezk I, Abou‐Taleb A, et al. (2020) Congenital laryngomalacia: Is it an inflammatory disease? The role of vitamin D. The Laryngoscope 130(2): 448-453.
- Isaac A, Zhang H, Soon SR, Campbell S, El-Hakim H (2016) A systematic review of the evidence on spontaneous resolution of laryngomalacia and its symptoms. International journal of pediatric otorhinolaryngology 83: 78-83.
- Rathi A, Rathi S (2017) Relative imbalance as etiology of laryngomalacia–A New Theory. Medical Hypotheses 98: 38-41.
- Munson PD, Saad AG, El‐Jamal SM, Dai Y, Bower CM, Richter GT (2011) Submucosal nerve hypertrophy in congenital laryngomalacia. The Laryngoscope 121(3): 627-629.
- Thompson DM (2007) Abnormal sensorimotor integrative function of the larynx in congenital laryngomalacia: a new theory of etiology. The Laryngoscope 117(S114): 1-33.
- De Vita F, Lauretani F, Bauer J, Bautmans I, Shardell M, Cherubini A, et al. (2014) Relationship between vitamin D and inflammatory markers in older individuals. Age 36(4): 1-13.
- Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, et al. (2016) Vitamin D deficiency in Europe: pandemic? The American journal of clinical nutrition 103(4): 1033-1044.
- Gholami F, Moradi G, Zareei B, Rasouli MA, Nikkhoo B, Roshani D, et al. (2019) The association between circulating 25-hydroxyvitamin D and cardiovascular diseases: A meta-analysis of prospective cohort studies. BMC Cardiovascular Disorders 19(1): 1-11.
- Sharawat IK, Dawman L (2019) Bone mineral density and its correlation with vitamin D status in healthy school-going children of Western India. Archives of osteoporosis 14(1): 13.
- Antonucci R, Locci C, Clemente MG, Chicconi E, Antonucci L (2018) Vitamin D deficiency in childhood: old lessons and current challenges. Journal of Pediatric Endocrinology and Metabolism 31(3): 247-260.
- Sharma D, Pandita A, Pratap OT, Murki S (2014) Laryngospasm, and neonatal seizure due to hypocalcemia and vitamin D deficiency: an emergency condition in NICU and challenge to the neonatologist bcr2014206795.
- Halter man JS, Smith SA (1998) Hypocalcemia and stridor: an unusual presentation of vitamin D-deficient rickets. The Journal of emergency medicine 16(1): 41-43.
- Bassiouni H, Aly H, Zaky K, Abaza N, Bardin T (2017) Probing the relation between vitamin D deficiency and progression of medial femoro-tibial osteoarthritis of the knee. Current rheumatology reviews 13(1): 65-71.
- Mabey T, Honsawek S (2015) Role of vitamin D in osteoarthritis: molecular, cellular, and clinical perspectives. International Journal of Endocrinology 2015: 383913.
- Wacker M, Holick MF (2013) Vitamin D-effects on skeletal and extra skeletal health and the need for supplementation. Nutrients 5(1): 111-148.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism 96(7): 1911-1930.
- Bozkurt HB, Çelik M (2021) Investigation of the serum vitamin D level in infants followed up with the diagnosis of laryngomalacia: a case-control study. European Archives of Oto-Rhino-Laryngology 278(3): 733-739.
- Schipani E, Provot S (2003) PTHrP, PTH, and the PTH/PTHrP receptor in endochondral bone development. Birth Defects Research Part C: Embryo Today 69(4): 352-362.
- Krishnan Y, Grodzinsky AJ (2018) Cartilage diseases. Matrix Biology 71: 51-69.
- Jin X, Jones G, Cicuttini F, Wluka A, Zhu Z, Han W, et al. (2016) Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: A Randomized Clinical Trial. Jama 315(10): 1005-1013.
- Veronese N, Maggi S, Noale M, De Rui M, Bolzetta F, Zambon S, et al. (2015) Serum 25-hydroxyvitamin D and osteoarthritis in older people: the Progetto Veneto Anziani study. Rejuvenation Research 18(6): 543-553.
- Hollis BW, Wagner CL (2013) Vitamin D and pregnancy: skeletal effects, nonskeletal effects, and birth outcomes. Calcified Tiss ue International 92(2): 128-139.
- Hossein Nezhad A, Holick MF, (2013) Vitamin D for health: a global perspective. Mayo clinic proceedings 88(7): 720-755.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...