Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Research Article(ISSN: 2641-1709) 
Efficacy of a Medical Device Based on Plant Extracts for the Symptomatic Treatment of Cough in Children and Adults. A Clinical Study Volume 7 - Issue 3
Gaetano Bottaro1, Benedetta Veruska Costanzo2, Filippo Palermo3, Tiziana Pecora4* and Vincenzo Perciavalle5
- 1Family Pediatrician, Catania, Italy
- 2General Practitioner, Catania, Italy
- 3Professor of Statistics, University of Catania, Italy
- 4Project Innovation Manager, Labomar SPA, Istrana (TV), Italy
- 5Professor of Physiology, University Kore of Enna, Italy
Received:October 23, 2021; Published: November 08, 2021
Corresponding author: Tiziana Pecora, Project Innovation Manager, Labomar SPA, Istrana (TV), Italy
DOI: 10.32474/SJO.2021.07.000265
Abstract
Cough is one of the most common medical situations that leads to seek medical attention. The present study was carried out
to evaluate the efficacy and tolerability in the treatment of cough in adults and children of a sugar-free syrup containing glycerol
and extracts from roots of Althaea officinalis and from leaves of Plantago lanceolata (FTP 65 Cough syrup sugar free: Manufacturer
Labomar SPA). One hundred and twenty subjects (60 children and 60 adults) were recruited. Participants received for 7 days one
of the following dosage schedules:
a) Adult: “ Cough Syrup REF FTP 65” in single-dose containers of 10 ml, to be taken daily in 3 administrations
b) Children: “ Cough Syrup REF FTP 65” with a daily dosage of 10 ml/day for 2–6-year-old children, and of 20 ml/day, for
7–12-year-old children. The survey included three visits: a first visit (V0) of enrollment, a second visit (V1) after 3 days from the V0
and a third visit (V2) carried out after 7 days of therapy. The results of this study confirm the evidence that the medical device Cough
Syrup REF FTP 65 may represent a valid choice as a treatment for coughing in adults and children
Keywords: Cough; mucociliary clearance; Althaea officinalis; Plantago lanceolata; moisturizing film
Introduction
Cough is one of the most common medical situations that leads
to seek medical attention. It is an archaic reflex act that aims to
protect the respiratory tract from foreign materials. Despite its
frequency, there are still no objective methods to quantify the cough
whose assessment remains, therefore, eminently subjective. Due to
the vagueness of the nature of this symptom, together with a strong
impact on the quality of life, the absence of objective tools and
the possibility that it is expression of an insidious pathology, the
cough should be considered and cured as a major problem until a
harmless etiology is identified. The cough is a reflex act, essentially
uncontrollable, capable to facilitate the mucociliary clearance and
to determine the removal of excess secretions from the airways. The
mucociliary clearance depends on the presence on inner surface of
the respiratory tree, from the trachea to the terminal bronchioles,
of a ciliated respiratory epithelium. Every single epithelial cell has
about 200 cilia (each cilium is about 7 μm in length) that protrude
towards the lumen and that constantly beat at a speed of 10-20 Hz.
The cilia are surrounded by a layer of periciliary fluid formed
by a deeper, very fluid layer, in turn dominated by a mucous
layer, together forming the epithelial lining fluid that, in addition
to keeping the epithelium moist, traps the particulate material
that has penetrated the respiratory tract. The cilia beat with a
coordinated movement directed towards the pharynx where the
transported mucus is swallowed or eliminated with the cough. An
effective mucociliary clearance depends on a number of factors,
including the number of cilia, their structure, the frequency with which they oscillate, and the quality of the mucus produced which
must be maintained at a correct humidity, temperature and acidity.
The cilia must be able to move freely in the layer of periciliar fluid
and when this is compromised, the mucus cannot be correctly
removed from the respiratory tract [1]. The cough reflex act
is characterized by the simultaneous closure of the glottis and
activation of an expiratory act with the resulting increase in the
intrathoracic pressure that can even exceed 300 mm Hg. The strong
increase in intrapulmonary pressure causes the violent expulsion
of the airway contents through the glottis into the pharyngeal
space and, therefore, out of the respiratory system. Due to the high
intensity of this process, in which the velocity of the expired air
can exceed 800 km/h, mucous secretions are detached from the
airway and expelled. In normal conditions, the cough is a protective
mechanism, but under certain circumstances can occur alterations
in the system’s physiology capable to generate situations that are
often unfavorable and exasperating for the patient.
The cough reflex is initiated by the activation of sensory
receptors located mainly, but not exclusively, in the pharynx, in
the larynx and along the entire tracheobronchial tree. Some of
these receptors are activated by chemical stimuli while others by
mechanical ones. Chemical type receptors, located mainly in the
respiratory tract, are activated by acids, heat and capsaicin-like
molecules that act on capsaicin type-1 receptors. The mechanical
type receptors are located not only in the respiratory tract but also
in the in the paranasal sinuses, eardrum, external auditory canal,
in pleura, pericardium, diaphragm, and stomach [2]. The different
types of sensory receptors are connected to the CNS through
afferent fibers that travel in the Vagus nerve (cranial nerve X) to the
respiratory centers located in the brainstem. The exact location of
the hypothetical cough center is not known, but it is likely that the
cough, rather than the effect of the activation of a single structure,
is the consequence of the modulation of the different respiratory
centers located in the brainstem [3]. Traditionally, the cough’s
mechanism is divided into 3 successive phases: the inspiratory
phase, the compression phase and the expiratory phase. The
first has the task of making a sufficient amount of air enter the
lung to produce an efficient cough. The compression phase is
characterized by the closure of the glottis and the simultaneous
contraction of the expiratory musculature that leads to a strong
increase in intrapulmonary pressure. Finally, the expiratory phase
is characterized by a rapid opening of the glottis resulting in an
explosive outflow of air. At the end of the exhalation, an inspiration
usually occurs to compensate for the developed hypoxia.
As mentioned above, in addition to the use of pharmacological
treatments based on antitussives, antihistamines or fluidizers,
cough can be treated by using plant extracts rich in polysaccharides.
In fact, they, with a purely mechanical action, form a moisturizing
and protective film [4] with a barrier effect that, on the one
hand, limits contact with irritating agents and, on the other one,
moisturizes the mucus so favoring a physiological expectoration.
The present medical device is a sugar-free syrup containing glycerol
and polysaccharide-rich aqueous extracts from roots of Althaea
officinalis and from leaves of Plantago lanceolata. Althaea officinalis
is traditionally used for the treatment of cough and inflammation of
the upper respiratory tract. Its roots are known to contain relatively
high mucilage content that makes this herb excellent demulcent,
emollient, expectorant [5]. Plantago lanceolata is indicated for
the treatment of cough for the presence of components such as
mucilages with an emollient and soothing action [6]. Glycerol or
glycerine is an ingredient found in many cough preparations for
its lubricant, emollient, and humectant properties [7]. The aim of
the present study was to confirm the efficacy and tolerability of the
syrup in the treatment of cough in adults and children from 2 years
of age.
Materials and Methods
Study design
In the period between March and May 2019, a multicenter postmarketing
study was carried out. The study was conducted at two
primary care clinics, the first of general practitioner and the second
of family pediatrician, involving: adults, males and females, with
an age between 18 and 65 years, and children, male and female,
with an ages between 2 and 12 years. A total of 120 subjects were
recruited: 60 children and 60 adults. The used product was Cough
Syrup REF FTP 65 (Labomar SPA, Istrana , TV, Italy) a medical device
already available on the European market under different brand
names and composed of extract of Plantago lanceolata, extract
of Althaea officinalis, vegetable glycerin, thyme extract, sorbitol,
water, xanthan gum, natural flavors, citric acid, potassium sorbate,
sugar-free and gluten-free. The experimental protocol “Study Code:
SFT2018” was approved by the Ethical Committee “Catania 2”, in
the session of 11/13/2018. All adult subjects signed the Informed
Consent approved by the Ethics Committee, while for children the
Informed Consent was signed by both parents and children over
5-year-old. After Informed Consent and basic evaluations, patients,
considered suitable for enrollment in the study, received one of the
following dosage schedules according to the instructions for use of
manufacturer:
a) Adult: “Cough Syrup REF FTP 65” in single-dose containers of
10 ml, to be taken daily in 3 administrations.
b) Children: “Cough Syrup REF FTP 65” with a daily dosage of
10 ml/day for 2–6-year-old children, and of 20 ml/day, for
7–12-year-old children.
The overall duration of the survey for each subject was seven
days.
Inclusion criteria
Subjects were admitted to the survey:
a. Females and males’ adults, suffering from cough associated
with sore throat or upper respiratory tract infections.
b. Females and males’ children, suffering from cough also
associated with sore throat or upper respiratory tract
infections
Exclusion criteria
Subjects were not included in the survey if:
a. Presenting contraindications indicated in the instructions for
use of the product “ Cough Syrup REF FTP 65”;
b. Presenting presumed or ascertained hypersensitivity to one or
more of the ingredients present in the formulation
c. Having a history of anaphylaxis or allergic reactions in general
or serious food intolerances, which the doctor may deem
relevant for inclusion in the study with concomitant conditions
that do not guarantee participation / participation in the study
according to the investigator’s opinion.
d. Were children affected by chronic pathologies with serious
respiratory compromises (cystic fibrosis, Spinal muscular
atrophy, dystrophies, cerebral palsy or oncological
pathologies).
e. Were adults with an overly complicated clinical picture for
which the investigating doctor believes that participation in
the study may be in some way harmful.
f. Were adults and children already in therapy with other
preparations for cough treatment in acute respiratory
infections.
Concomitant therapies
The subject had to follow an adequate diet; subjects in therapy with other cough preparations including cough suppressants and mucolytics were not included in the study. Any assumption of concurrent permitted and non-permitted drugs was reported in the Data Collection Form (DCF) and used for the final evaluation.
Study procedures
The survey included three visits. A first visit (V0) of enrollment,
a second visit (V1) carried out after 3 days from the V0 and a third
visit (V2) carried out after 7 days of therapy, i.e., at the end of the
study therapy.
V0 (enrollment): first enrollment visits and compilation of the
SRD:
a. explanation of the investigation to the subject if adult or to the
parent / legal representative and obtaining written Informed
Consent before each investigation procedure.
b. medical history and verification of inclusion and exclusion
criteria and clinical evaluation.
c. evaluation of the cough by using a questionnaire.
d. assessment of associated symptoms:
e. for adults: general conditions (poor, satisfactory good), fatigue,
vomiting, hoarseness, difficulty breathing, chest pain, vertigo
and urine leakage; all these parameters were evaluated in
mild, moderate, severe.
f. for children: general conditions (poor, satisfactory good),
fever (Celsius degrees, °C), headache, vomiting, hoarseness,
stuffy nose, ear pain, assessed in mild, moderate, severe, and
respiratory sounds (wheezes, rhonchi, and rales).
g. assessment of psychophysical well-being for adults, with
questions as: do you feel depressed? Do you feel irritated? Do
you feel tired? Do you consider yourself sick?
h. evaluation of daily activities such as: loss of school days, loss
of days of work of parents, reduction of appetite and sense of
discomfort.
i. any concomitant therapy and the type of drug used were
recorded.
j. setting of therapy with “ Cough Syrup REF FTP 65” and delivery
of product.
k. programming of visit 1 (V1).
V1: second visit:
a) review of inclusion and exclusion criteria.
b) evaluation of the symptom cough based on the questionnaire.
c) clinical evaluation and associated symptoms.
d) evaluation of the parameters of psychophysical wellbeing for
adults and those of daily activities for children.
e) registration of any adverse effects.
V2: final visit, conclusion of the study
a) review of inclusion and exclusion criteria.
b) evaluation of the symptom cough based on the questionnaire.
c) assessment of the patient’s general condition.
d) evaluation of efficacy, tolerability and compliance of the
medical device.
e) assessment of patient satisfaction.
f) evaluation of the parameters of psychophysical wellbeing for
adults and those of daily activities for children.
g) assessment of any adverse effects incurred.
h) completion of the end of study procedures.
Study endpoints
The primary endpoint was the evaluation of the efficacy of the medical device Cough Syrup REF FTP 65 to reduce the symptom cough in adults and children with acute respiratory diseases. To assess the cough’s intensity and its impact on the patient’s daily life we used a questionnaire taken from validated cough measurement instruments such as cough scales (Table 1). The result was given by the variation from baseline (V0) to the intermediate visit (V1) and at the end of the treatment (V2). The secondary endpoints included the evaluation of the clinical status, the evaluation of efficacy and tolerability of the medical device Cough Syrup REF FTP 65 obtained from the variation from baseline (V0) to the intermediate visit (V1) and at the end of treatment (V2).
Data analysis
The sample used was considered adequate for the purpose of the study, namely: observation and description of the efficacy and safety characteristics of the product under study in the postmarketing follow-up. The intention-to-treat population was used for the analysis of the raw data. Comparisons between the groups, openly treated, were of a descriptive nature and data were reported in terms of means and standard deviations (SD) for quantitative variables and in terms of absolute frequencies and percentages for qualitative variables. To detect significant differences in changes in primary and secondary efficacy endpoints from baseline (V0) to the end of treatment (V2) the exact Fisher test, Chi square test with Yates’s correction, McNemar test and Mann-Witney test were used.
Results
Children
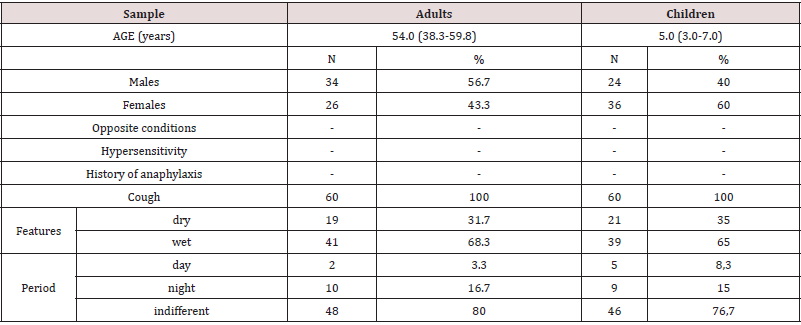
The 60 children had a median age of 5 years (range 3-7 years), 24 were males (40%) and 36 females (60%); all had coughs and had no contraindications to the use of medical device Cough Syrup REF FTP 65, nor conditions that hindered the taking. The cough was dry in 21 (35%) and wet in 39 (65%) children. Only 5 (8.3%) children had daytime cough, 9 (15%) had nocturnal cough, all the other 46 (76.7%) children had either daytime or night cough. Concerning the associated symptoms, at the time of diagnosis (V0) 40 children (66.7%) had poor general conditions, 17 (28.3%) satisfactory conditions, 3 (5%) very poor conditions and non-good conditions. Forty-three children (71.7%) had fever with a median value of 38 °C (38 - 38.5 °C); 51 children (64.7%) had headaches, most of them mild (33, 64.7%), 18 moderate (35.3%); 6 children (10%) had mild vomiting. Fifty-two children (86.7%) had hoarseness, 36 (69.2%) moderate, 13 (25%) mild and only 3 (5.8%) severe. Fiftythree children (88.3%) had coryza, out of which 11 (20.8%) mild, 38 (71.7%) moderate, 4 (7.5%) severe. Eight children (13.3%) had ear pain, out of which the majority were mild (6, 75%), 1 moderate (12.5%) and 1 severe (12.5%). Finally, 54 children (90%) had positive respiratory sounds, 7 children (13%) had wheezing, 31 (57.4%) Ronchi and 16 (29.6%) rales (Table 2).
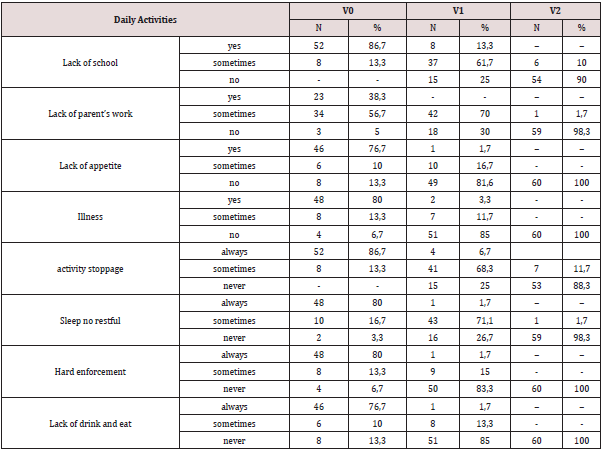
As regards the influence on activities, at the time of diagnosis only 4 children (6.7%) declared that cough did not affect their activities while 56 children (93.3%) sometimes felt compelled to interrupt them. Fifty-two children (86.7%) stated that sometimes they did not have a restful sleep, 2 (3.3%) always, 6 (10%) never; 35 children (48.3%) stated that sometimes he had difficulty concentrating, 10 (16.7%) always, 15 (25%) never. Thirty-five children (58.3%) declared that, when sick, they always stopped eating, 15 (25%) sometimes, 10 (16.7%) ever (Table 3). As for daily activities, at the time of diagnosis 52 children (86.7%) had constant loss of school days and 8 (13.3%) only sometimes. The parents of 23 children had always lost working days, 34 (56.7%) sometimes, only the parents of 3 children (5%) had not lost working days; 46 children (76.7%) had always lost their appetite, 6 (10%) sometimes, 7 (13.3%) never; finally, 48 children (80%) always had a sense of malaise, 8 (13.3%) sometimes, 4 (6.7%) never.
At the time of the first visit, in addition to the product being
tested, 29 children (47.1%) received another drug, most often
salbutamol (13 children, 44.8%), 6 children (10%) received an
antibiotic, 6 children (20.7%) the combination paracetamolchlorphenamine,
1 child (3.4%) beclomethasone for inhalation,
1 child (3.4%) cetirizine and, finally, 3 children (10.3%) other
preparations for cough. The endpoints evaluation showed that
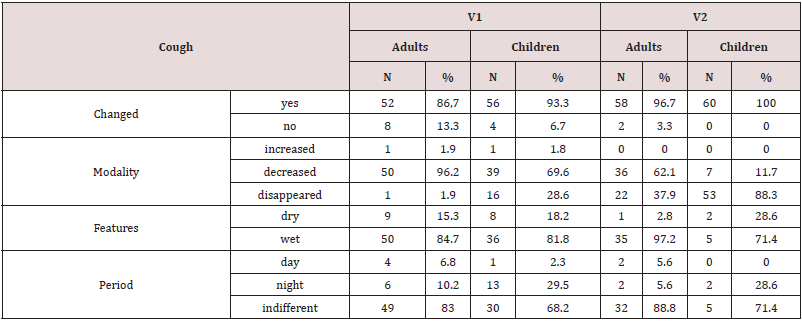
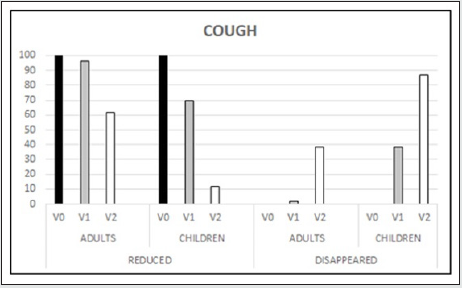
already at V1, after only 3 days of therapy, 56 children (93.3%)
showed a change in cough and only 4 (6.7%) had no change; those
in which the coughing had changed, 39 children (69.6%) had decrease, in 16 (28.6%) cough disappeared and only in 1 (1.8%)
cough increased. Concerning the characteristics, the cough was
kept wet in 36 children (81.8%) and dry in 8 (18.2 &); the cough
remained both diurnal and nocturnal in 30 (68.2%), while 13
(29.5%) still presented it nocturnal and only 1 (2.3%) diurnal. At the
final visit all the children (100%) had modified cough, in 7 (11.7%)
cough decreased, while in the other 53 (88.3%) cough disappeared.
The characteristics had remained the same: predominantly wet
(71.4%) and indifferent (71.4%). As for the associated symptoms,
these showed, already at V1, an improving trend, with the exception
of the general conditions that remained unchanged with respect to
the initial visit. On the contrary, fever persisted in only 3 children
(5%), a mild headache was present only in 12 (20%), vomiting only
in 1 (1.7%), while hoarseness persisted in 15 (25 %). In 19 children
(31.7%) persisted the coryza, out of which 16 (84.2) was mild; in 3
(5%) persisted ear pain and in 12 (20 %) respiratory sounds, more
often ronchi (10, 83.4%). At V2 the improvement was evident with
good general conditions in all 60 children (100%); only 1 (1.7%)
persisted headache, none vomited, only 2 (3.3%) had hoarseness,
2 (2.2%) coryza, none had more ear pain, and none had abnormal
respiratory sounds.
As for the influence on daily activities, already at V1 only 4
(6.7%) and none at V2, had been forced to interrupt them. Fortyone
(68.3%) were sometimes at V1 and 7 (11.7%) at V2, 15 (25%)
and 53 (88.3%) at V2 were no longer; only 1 (1.7%) and none at
V2, had no restful sleep, 43 (71.7%) had sometimes not and 16
(26.7%) had never at V1 and only 1 (1.7%) at V2, mind the other
59 (98.3%) never. Fifty children (83.7%) at V1 and all 60 (100%)
at V2 had no more difficulty in concentrating; only 1 (1.7%) always
had it and 9 (15%) sometimes at V1. Finally, already 51 children
(85%) had no more difficulties in eating and drinking in V1 and
15 (25%) in V2, 10 (16.7%) and 45 (76.7%) in V2 were no more;
11 subjects (6.7%) and only 1 (1.7%) always had difficulties and 8
(13.3%) sometimes, in V2 nobody had any more. As far as the daily
functions, already at V1 were concerned, only 8 (13.3%) and none
at V2, had lost their schooling, 37 (61.7%) had lost it sometimes
at V1 and 6 (10%) at V2, 15 (25%) and 54 (90%) at V2 had not
lost it anymore. In 42 children (70%) at V1 and only 1 (1.7%) at
V2, the parents had sometimes lost their job, while 18 (30%) at V1
and 59 (98.3%) at V2, had not lost it anymore. Only 1 child (1.7%)
and none at V2 had a reduction in appetite, 10 children (16.7%) at
V1 and none at V2 had sometimes a reduction in appetite, while
49 children (81.6%) at V1 and all (100%) at V2 had no reduction
in appetite. Finally, only 2 children (3.3%) at V1 and none at V2
had a reduction in appetite, 7 (11.7%) at V1 and none at V2 had
sometimes a reduction in appetite, 51 children (85%) at V1 and
none at V2 had a reduction in appetite.
Nineteen children out of the initial 29 had maintained the
associated drug therapy, as was logical to think, but at V2 still 10
children were taking an associated drug.
In terms of efficacy, 53 children (88.3%) assessed it as good at
V1, 2 children (3.3%) as satisfactory and 5 (8.4&) as excellent, while
at V2 all 60 children (100%) assessed it as excellent. Tolerability
was considered good by 59 children (98.3%) already at V1, only 1
(1.7%) satisfactory, while at V2, all 60 children (100%) considered
it very good. Only one child (1.7%) was agitated when taking the
syrup, which lasted a few days during the therapy, which did not
need to be suspended, and disappeared immediately after the
suspension of the therapy. All subjects took all the doses (8 to V1
and 14 to V2) and followed the therapy. 25 children (41.7%) found
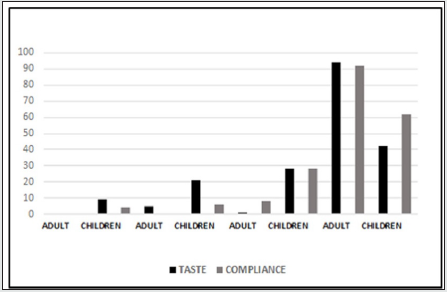
the taste of the syrup very good, 17 (28.3%) good, 13 (21.7%)
bad and 5 (8.3%) very bad; 38 children (63.3%) considered the
syrup very easy to swallow, 17 (28.3%) easy, 3 (5.1%) difficult, 2
(3.3%) very difficult. The statistical analysis showed high statistical
significance (chi2 p<0.001) for all the variables already considered
at the intermediate visit and especially at the final visit. The
following associated symptoms also showed a statistically highly
significant reduction (p<0.001) in McNamar’s test at both V1 and
V2: fever, headache, hoarseness, coryza and chest pain; at V2 the
reduction was statistically significant for vomiting (p<0.05) and
otalgia (p<0.01) because they already started from a small number
of subjects (Figures 1 & 2).
Adults
The 60 adults had a median age of 54 years (range 38-60 years),
34 were males (57%) and 26 females (43%), all had coughs and
had neither contraindications to the preparation nor conditions
that hindered its intake. The cough was dry in 19 subjects (32%),
wet in 41 (68%) subjects. Only 2 (3%) adults had daytime cough,
10 (17%) had nighttime cough, all the other 48 (80%) had both
daytime and nighttime cough. Concerning the associated symptoms,
at the time of diagnosis 31 (51.7%) had poor general conditions, 24
(40%) satisfactory, 1 (1.7%) poor and 4 (6.6%) good. Fifty-three
adults (88.3%) experienced fatigue, although the majority (39,
73.6%) experienced mild fatigue; 42 adults (70%) suffered from
headache, the majority of which were mild (36, 85.7%), 6 subjects
(10%) had vomiting and 9 (15%) hoarseness. Forty-two subjects
(70%) had difficulty breathing, of which 22 (52.4%) were mild;
40 subjects (66.7%) had chest pain, the majority of which were
mild (25, 62.6%); only 3 subjects (5%) had dizziness and 6 (10%)
slight loss of urine (Table 2). As regards the influence on physical
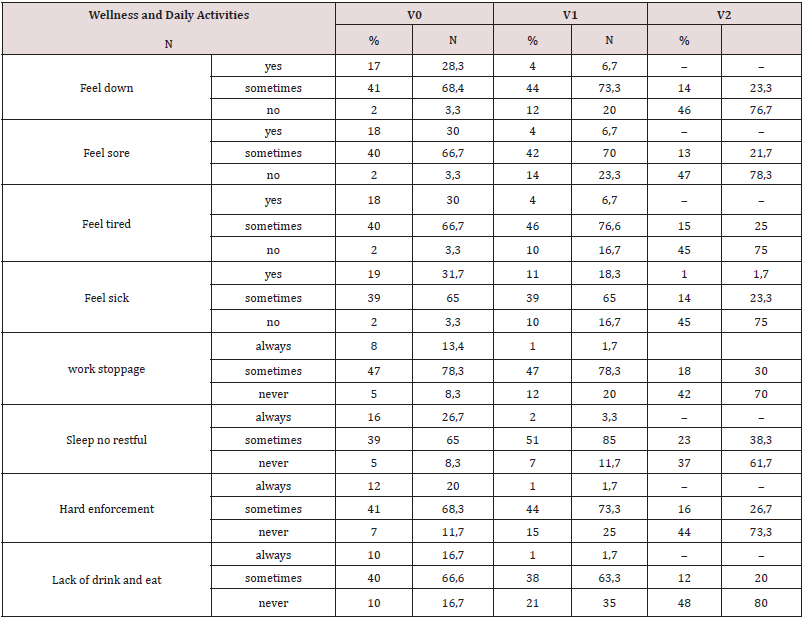
well-being, at the time of diagnosis 41 subjects (68.3%) sometimes
felt depressed, 17 (28.3%) always felt depressed, 2 (3.3%) never.
Forty subjects (66.7%) sometimes felt irritated, 18 (30%) always, 2
(3.3%) never; 40 subjects (66.7%) sometimes felt tired, 18 (30%)
always, 2 (3.3%) never; 39 subjects (65%) sometimes felt sick, 19
(31.7%) always, 2 (3.3%) never.
Concerning daily activities, at the time of diagnosis 47 subjects
(78.3%) sometimes interrupted their work, 8 (13.4%) always, 5
(8.3%) never; 39 subjects (65%) had sometimes no restful sleep,
16 (26.7%) always, 5 (8.3%) never. Forty-one subjects (68.3%)
sometimes had difficulty concentrating, 12 (20%) always, 7 (11.7%)
never; finally, 40 subjects (66.7%) sometimes stopped drinking and
eating, 10 (16.7%) always, 10 (16.7%) never (Table 4). At the first
visit and in conjunction with the investigational product 21 subjects
(35%) received another drug, more often an antibiotic (8, 13.3%),
than a steroid (6, 10%), someone an antihistamine. The evaluation
of endpoints showed that already at V1, after 3 days of therapy, 52
subjects (86.7%) showed a modification of the cough and 8 (13.3%)
a stationarity; of those in which it was modified, 50 (96.2%) had
shown a decrease, 1 (1.9%) disappeared and 1 (1.9%) increased.
As far as its characteristics were concerned, the cough remained
wet (84.7%) and indifferent between day and night (83%). At the
final visit the same trend was maintained with 58 (96.7%) subjects
in which it had changed and of these 36 (62.1%) showed a decrease
and 22 (37.9%) disappeared. The characteristics had remained the
same: mainly wet (97.2%) and indifferent (88.8%).
Regarding the associated symptoms, they showed, already at
V1, an improving trend. General conditions were satisfactory in
43 (71.7%) and good in 13 (21.7%), headache was present only
in 19 (31.7%), vomiting and hoarseness only in 3 (5%), difficulty
in breathing persisted in 34 (56.7%) subjects and chest pain in 25
(41.7%), no one had dizziness and only 2 (3.3%) loss of urine. At V2
the improvement was evident with good general conditions in 55
(91.7%), only 4 (6.7%) had headaches, no one vomited, only 3 (5%)
had hoarseness, 16 (23.7%) had difficulty breathing, 3 (5%) chest
pain and only 1 (1.7%) dizziness.
As for the influence on physical well-being, already at V1 only
4 (6.7%) and none at V2, still felt depressed, 44 (73.3%) were
sometimes depressed at V1 and 14 (23.3%) at V2, 12 (20%) and
46 (76.7%) at V2 were no longer. Only 4 (6.7%) and none at V2,
still felt irritated, 42 (70%) were sometimes at V1 and 13 (21.7%)
at V2, 14 (23.3%) and 47 (78.3%) at V2 were no longer irritated.
Only 4 (6.7%) and none at V2, still felt tired, 46 (76.6%) were
sometimes at V1 and 15 (25%) at V2, 10 (16.7%) and 45 (76.7%)
at V2 were no longer tired. Eleven subjects (6.7%) and only 1
(1.7%) at V2, still felt sick, 39 (65%) were sometimes at V1 and
14 (23.3%) at V2, 10 (16.7%) and 45 (75%) were no longer sick.
Regarding daily activities, already at V1, only 1 (1.7%) and none
at V2, had interrupted work, 47 (78.3%) had sometimes done so
at V1 and 18 (30%) at V2, 12 (20%) and 42 (70%) at V2 had not
interrupted it anymore. Only 2 participants (3.3%) and none at V2
had a restful sleep, 51 (85%) had sometimes at V1 and 23 (38.3%)
at V2, 7 (11.7%) and 37 (61.7%) at V2 had a restful sleep. Only one
participant (1.7%) and none at V2, had difficulty concentrating, 44
(73.3%) had it sometimes at V1 and 16 (26.7%) at V2, 15 (25%)
and 44 (73.3%) at V2 had it no more. Finally, only 1 subject (1.7%)
and none at V2, had stopped drinking and eating, 38 (63.3%) had it
sometimes at V1 and 12 (20%) at V2, 21 (35%) and 48 (80%) had
it no more.
Seventeen subjects out of the initial 21 maintained the
associated drug therapy, as was logical to think, but at V2 only 5
were still taking an associated drug. Concerning effectiveness of
treatment, 30 subjects (50%) assessed it as good at V1 and 6 (10%)
at V2, 27 (45%) assessed it as excellent at V1 and 53 (88.3%) at V2,
only 2 (3.3%) at V1 and 1 (1.7%) at V2 considered it satisfactory
and 1 subject (1.7%) at V1 considered it poor. Tolerability was
considered good by 14 subjects (23.3%) to V1 and 2 (3.3%) to V2,
excellent by 45 subjects (75%) to V1 and 56 subjects (93.4%) to V2,
only 1 (1.7%) to V1 and 2 (3.3%) considered it poor. All subjects
took all the doses (9 to V1 and 20 to V2) and followed the therapy.
57 subjects (95%) found the taste of the syrup very good, 1 (1.7%)
good and only 2 (3.3%) bad; 56 subjects (93.3%) considered
it very easy to swallow the syrup and 4 (6.7%) easy, nobody
found it difficult. The statistical analysis showed high statistical
significance (chi2 p<0.001) for all the variables considered already
at the intermediate visit and especially at the final visit. Also, the
associated symptoms showed statistically significant difference
to McNemar’s test already at V1 headache (p<0.001) and chest
pain (p<0.01), but a bit all at V2: headache (p<0.001), vomiting
(p<0.01), difficulty breathing (p<0.001), chest pain (p<0.001) and
loss of urine (p<0.01). Only hoarseness and dizziness did not show
significant differences, probably because they already started from
a small number of subjects (Figures 1 & 2).
Discussion
Coughing at any age has to be dealt with different approaches. It is always necessary to go back to the initial cause and remove it, either from infectious causes, identifying the agent in question and starting the specific therapy, you know allergic or from irritating substances by removing the cause (allergens, smoke, pollutants, etc.). However, at the same time, it is essential to improve the symptom, to improve the quality of life and bring daily benefits. If it can be useful to alleviate the course of an annoying cough that lasts a few days, it is always necessary to intervene when the cough is caused by an identified etiological agent [8]. A specific treatment of the cough symptom is of great importance both for the adult, because it improves the mood, the perception of health and the evaluation of one’s own state of health, and in the child, because seeing his or her own little cough creates fear and discomfort in the whole family. In any case, if the cough does not pass within a few days, it is necessary to initiate more specific diagnostic investigations to make a precise diagnosis. The treatment of the symptom cough has always relied on sedatives, i.e., substances that, by suppressing the cough reflex, prevent coughing [9]. Unfortunately, some act on the central nervous system and derive from morphine (codeine, etc.), others with central action do not derive from morphine (dextromethorphan, cloperastine, etc.), others are peripheral sedatives (levodropropizine, etc.), acting on sensory fibres afferent to the center of the cough. Many of these cough sedatives cannot be used in children under 2 years of age and in any case both in older children and adults, they are all of dubious efficacy and have presented, depending on the different points of action, important side effects, mainly represented by restlessness and drowsiness [10-15]. Banned expectorant syrups, never indicated in children under 12 years of age and working badly in adults with few exceptions [16], the alternative is to use natural preparations. Most of the preparations did not fail the most important test of effectiveness and showed to reduce the discomfort of coughing. The active ingredients used for these preparations have been many over time, starting with thyme, ivy, etc., all plants that nature makes available to us to soothe the problems of the first airways and in cases of coughing. In this context is inserted the medical device object of the present clinical investigation which, thanks to its components (glycerol, marshmallow and plantain), has a calming action on the irritation and protective action of the respiratory tract mucosa. The results of the present study showed that the medical device used by us is highly effective in improving the cough symptom, even after only 3 days of therapy in the adult (86.7%) and after 4 days of therapy in the child (93.3%). In addition to the change of cough all symptoms improve after the first days of therapy and this allows a rapid resumption of daily functions, work activities and overall perception of health status. The combined action of Alhtea, Plantago and glycerol has allowed our device to act completely on all kind of cough (wet, dry) presented by both adults and children, resulting effective. It is worth noting that a recent review confirmed the efficacy of Alhtea officinalis extracts alone in treatment of dry cough, while in combination with other extracts improved all kinds of cough [17]. The cough is a symptom that is very annoying to the patient and his family, since it is linked to diseases important for severity and danger, its improvement is therefore able to determine serenity and tranquility important for a good course of the disease. In any case, a product, be it a drug or a device, must be not only effective but also well tolerated and agreeable. In the present study, we have noticed differences between adults and children. Adults consider Cough Syrup REF FTP 65 almost unanimously good/very good (97%), children a little less (70%). Only one child presented a side effect (agitation) which, in any case, was mild and did not require therapy to be discontinued.
Conclusions
The results of this study confirm the evidence that the medical device Cough Syrup REF FTP 65, with its content in natural functional components and its efficacy and safety profile, may represent a valid choice as a treatment for coughing in adults and children. It allows reducing the traditional therapy, with a faster return to school and to normal, working life of adults. In addition, the good taste and ease of administration of the product make it practical and easily accepted.
Statement of Ethi
All procedures in this study were performed in accordance with the of the Declaration of Helsinki and were approved by the Ethical Committee “Catania 2” of Catania (Italy).
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study received no financial support
Author Contributions
Vincenzo Perciavalle is the corresponding authors of this article. All authors planned and designed the study; Gaetano Bottaro and Benedetta Veruska Costanzo performed the experiment. Tiziana Percora and Filippo Palermo are responsible for data collection. All authors contributed to the interpretation of the analysis. Vincenzo Perciavalle wrote the paper, and all authors critically revised the manuscript.
References
- Stanke F (2015) The contribution of the airway epithelial cell to host defense. Mediators of Inflammation 2015: 463016.
- Ebihara S, Izukura H, Miyagi M, Okuni I, Sekiya H, Ebihara T (2016) Chemical senses affecting cough and swallowing. Current Pharmaceutical Design 22: 2285-2289.
- Mutolo D (2019) Brainstem mechanisms underlying the cough reflex and its regulation. Respiratory Physiology & Neurobiology 243: 60-76.
- Franz G (1989) Polysaccharides in Pharmacy: current applications and future concepts. Planta Medica 55: 493-497.
- Sutovská M, Nosálová G, Sutovský J, Franová S, Prisenznáková L, Capek P (2009) Possible mechanisms of dose-dependent cough suppressive effect of Althaea officinalis rhamnogalacturonan in guinea pigs test system. nternational Journal of Biological Macromolecules 45: 27-32.
- Wegener T, Kraft K (1999) Plantain (Plantago lanceolata L.): anti-inflammatory action in upper respiratory tract infections [Article in German] Wiener klinische Wochenschrift 14: 211-216.
- Eccles R, Mallefet P (2017) Soothing properties of glycerol in cough syrups for acute cough due to common cold. Pharmacy (Basel) 5(1) p: 4.
- Kaplan AG (2019) Chronic cough in adults: make the diagnosis and make a difference. Pulmonary Therapy 5: 11-21.
- Wang K, Bettiol S, Thompson MJ, Roberts NW, Perera R, Heneghan CJ, Harnden A (2014) Symptomatic treatment of the cough in whooping cough. Cochrane Database Systematic Reviews 22(9): CD003257.
- Kurth W (1978) Secure therapeutic effectiveness of the traditional antitussive agent Mintetten in a double-blind study [Gesicherte therapeutische wirksamkeit des traditionellen antitussivums mintetten im oppelblindversuch]. Medizinische Welt 29: 1906–1909.
- Banderali G, Riva E, Fiocchi A, Cordaro CI, Giovannin M (1995) Efficacy and tolerability of levodropropizine and dropropizine in children with non-productive cough. Journal of International Medical Research 23: 175–183.
- Freestone C, Eccles R (1997) Assessment of the antitussive efficacy of codeine in cough associated with common cold. Journal of Pharmacy and Pharmacology 49: 1045–1049.
- Gunn VL, Taha SH, Liebelt EL, Serwint JR (2001) Toxicity of over-the-counter cough and cold medications. Pediatrics 108(3): e52.
- Kelly LF (2004) Pediatric cough and cold preparations. Pediatrics in Review 25: 115–123.
- Centers for Disease Control and Prevention (2005) Infant death associated with cough and cold medications - two states. Morbidity and Mortality Weekly Report 56: 1–4.
- Smith SM, Schroeder K, Fahey T (2014) Over-the-counter (OTC) medications for acute cough in children and adults in community settings. Cochrane Database Systematic Reviews (11): CD008131.
- Mahboubi M (2020) Marsh Mallow (Althaea officinalis L.) and its potency in the treatment of cough. Complementary Medicine Research 27(3): 174-183.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...