Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Research Article(ISSN: 2641-1709) 
Effect of Epipharyngeal Abrasive Therapy on Long COVID with Chronic Epipharyngitis Volume 8 - Issue 5
Hirobumi Ito*
- Department of ENT, Ito ENT Clinic, Japan
Received: September 06, 2022; Published: September 16, 2022
Corresponding author: Hirobumi Ito, Ito ENT Clinic, 4-13-3 Yatsuya Headquarters Building 3rd Floor, Maebarahigashi, Funabashi City, Chiba Prefecture 274-0824, Japan
DOI: 10.32474/SJO.2022.08.000300
Abstract
Introduction: Currently, there is no established treatment for Long COVID (LC), but epipharyngeal abrasive therapy (EAT) has been reported to be useful in cases of complicated chronic epipharyngitis. In this study, we performed EAT on LC patients with chronic epipharyngitis and investigated the effects of EAT on subjective symptoms, examination findings, and autonomic nervous system function.
Methods: Epipharyngeal abrasive therapy (EAT) was performed on 31 patients with Long COVID with chronic epipharyngitis. We recorded changes in subjective symptoms using the EAT score and changes in bleeding during abrasion using the bleeding score. Heart rate variability analysis was performed before and after treatment to assess autonomic function.
Results: Bleeding and EAT scores improved significantly over time (p < 0.01), but the autonomic function test did not show any significant improvement. Noting that, the EAT score improved earlier than the bleeding score did.
Conclusions: Assuming that the improvement in autonomic function would take time, we consider EAT to be a useful treatment for patients with LC who have chronic epipharyngitis.
Keywords: long COVID; EAT; EAT score; bleeding score; heart rate variability analysis
Abbreviations: PVFS: Post Viral Fatigue Syndrome; LC: long COVID; ME/CFS: Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; EAT: Epipharyngeal Abrasive Therapy
Introduction
Post viral fatigue syndrome (PVFS) is a long-standing syndrome in which patients suffer from the following conditions: low-grade fever, headache, weakness, impaired thinking, and neuropsychiatric symptoms such as depression [1]. Particular signs, including respiratory distress, malaise, olfactory and taste disturbance, hair loss, headache, and poor concentration, may persist for several months or longer after COVID-19 infection; such manifestations are reported as long COVID (LC) [2]. LC can cause a variety of prolonged symptoms not only in the respiratory tract but also in cranial nerves and other areas [3]. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a condition based on the modulation of the nervous, endocrine, and immune systems, is a brain dysfunction caused by various cytokines induced by viral reactivation and chronic infection [4]. Its pathogenesis is believed to be related to genetic and environmental factors, including physical and mental distress [5].
Outbreaks of ME/CFS have historically, occurred after viral diseases epidemics; thus, it is feared that an increase in COVID-19 might result in a rise in the occurrence of ME/CFS in the future [6]. Epipharyngeal abrasive therapy (EAT) is generally used to cure chronic epipharyngitis [7], which is common in LC. In this context, many studies have reported the effectiveness of EAT as a treatment for LC [8,9]. Moreover, EAT has been reported to be effective in treating ME/CFS [10]. Therefore, it may be worthwhile to prevent the onset of ME/CFS through the application of EAT in patients with LC with chronic epipharyngitis. Until now, there has been no established treatment for LC. In this study, we performed EAT on patients with LC with chronic epipharyngitis and investigated the effects of EAT on subjective symptoms, objective findings, and autonomic function tests. Because of persistent COVID-19 infection worldwide, the number of LC cases is expected to continue to increase. Therefore, we believe there is an urgent need to elucidate the pathogenesis of LC and establish effective evaluation and treatment methods.
Materials and Methods
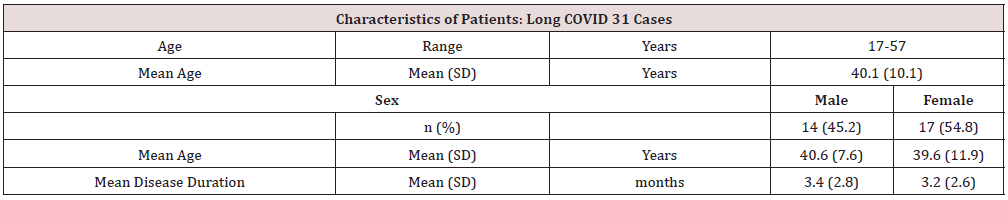
Patients with COVID-19 infection confirmed via polymerase chain reaction testing, complaining of abnormal nasopharyngeal sensation and posterior rhinorrhea, after at least 1 month of illness onset, were referred to our hospital for examination between May and September 2021. These patients were diagnosed with LC with residual symptoms of autonomic neuropathy such as fatigue, breathlessness, and light headedness. A total of 31 patients were included (14 males, 40.6 ± 7.6 years old, mean duration of disease 3.4 ± 2.8 months; 17 females, 39.6 ± 11.9 years old, mean duration of disease 3.2 ± 2.6 months). We identified 29 patients (13 males and 16 females) with chronic epipharyngitis according to Tanaka’s diagnostic criteria [11]. We evaluated these patients using the EAT questionnaire and hemorrhage findings during abrasion. We also evaluated 10 patients (3 males, 46.3 ± 10.5 years old, mean disease duration 5.0 ± 1.4 months; 7 females, 34.8 ± 14.1 years old, mean disease duration 2.7 ± 2.4 months) for autonomic function at the beginning of and during the treatment period.
We diagnosed and treated chronic epipharyngitis using a band-limited optical endoscope system (Pentax EPK-i7000 video processor, VNL11-J10 video scope with a 3.5-mm outer-diameter tip). We treated EAT via nasal abrasion using a Lutze cotton swab moistened with 1% zinc chloride solution, followed by oral abrasion with a Zermach pharyngeal crimp (Figure 1). We evaluated the patients’ subjective symptoms using the EAT questionnaire based on the numerical rating scale (NRS; Table 1). This questionnaire assesses local and general symptoms related to chronic epipharyngitis as follows: posterior rhinorrhea, nasopharyngeal irritation (abnormal pharynx, headache, stiff neck, etc.), nasalrelated symptoms (rhinorrhea, nasal obstruction, nasal irritation, etc.), Eustachian tube–related symptoms (otal obstruction, tinnitus, etc.), dizziness, voice-related symptoms (hoarseness, voice disorder, etc.), laryngeal irritation (cough, sore throat, etc.), and sleep apnea. We rated the following 10 items on a 6-point NRS scale: snoring, insomnia, daytime somnolence, gastrointestinal symptoms (acid reflux, heartburn, lethargy, etc.), and autonomic nervous system symptoms (fatigue, chronic fatigue, depression, etc.). The following scores were assigned to each item:
Figure 1: EAT treated by nasal abrasion with a Lutze swab soaked in 1% zinc chloride solution followed by oral abrasion with a Zermac pharyngeal crimp.
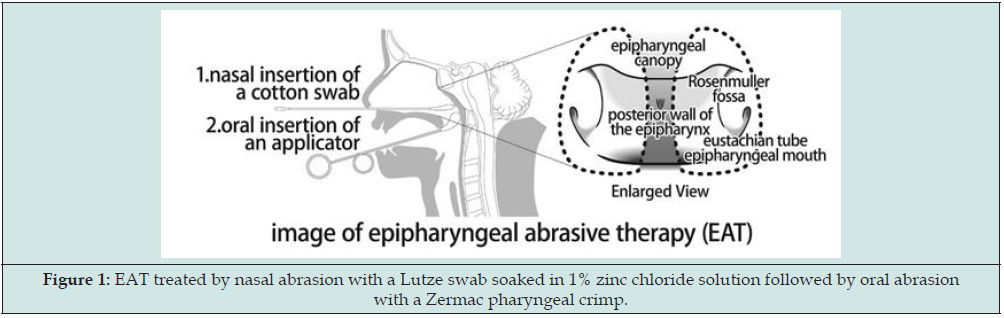
Table 1: Evaluation of subjective symptoms using the EAT questionnaire scored on a 6-point NRS scale. NRS: numerical rating scale.
Numerical Rating Scale (NRS)
0: Condition without subjective symptoms
1: Slightly/rarely concerned
2: A little/sometimes worrying
3: always sorry
4: Quite/almost bothers me, but I can put up with it
5: I’m so worried I can’t stand it.
a) 0 = no subjective symptoms,
b) 1 = slightly bothering,
c) 2 = sometimes bothering,
d) 3 = always bothering,
e) 4 = fairly bothering but tolerable, and
f) 5 = extremely bothering and intolerable.
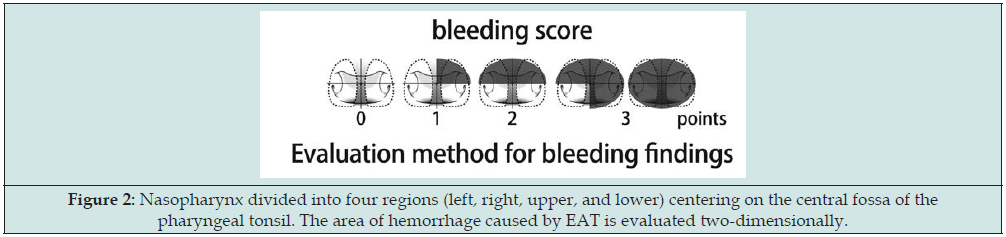
We computed the EAT score by summing the scores of each group with a range of 0–50 points. We defined the time of subjective symptom improvement as when the symptoms become absent or slightly bothersome (i.e., when the EAT score reached ≤ 10 points). Using endoscopic observation as an objective finding, we evaluated bleeding from the epipharyngeal mucosa at the time of abrasion. In general, bleeding is evaluated based on the degree of blood adherence to a cotton swab, but this method is not objective due to different adopted techniques and skills [11]. For this reason, quantitative evaluation of the degree of bleeding is considered to be difficult [12]. However, in this study, we evaluated the area spread of the bleeding site caused by abrasion in two dimensions. The epipharynx was subdivided into four (left, right, upper, and lower) centered with the central fossa of the pharyngeal tonsil. The bleeding score (0–3 points) was calculated from the obtained bleeding findings, with 3 points for severe bleeding, 2 points for moderate bleeding, 1 point for mild bleeding, and 0 points for no bleeding or barely blotting (Figure 2). We defined the time of improvement of other sensory findings as when the bleeding score improved to 0 (Figure 2).
Figure 2: Nasopharynx divided into four regions (left, right, upper, and lower) centering on the central fossa of the pharyngeal tonsil. The area of hemorrhage caused by EAT is evaluated two-dimensionally.
We performed autonomic function tests via heart rate variability analysis of autonomic reflexes induced by orthostatic stress using KIRITSU MEIJIN software for analysis (Crosswell Inc.). We evaluated the autonomic function for the following six items: high frequency (HF), low frequency (LF)/HF ratio (L/H), ⊿L/H, coefficient of variation on the R-R interval (CVRR), ⊿CVRR, and Kiritsu Master (KM) score [13]. Although heart rate is affected by respiration and circulation, periodic fluctuations occur in the electrocardiogram R-R interval. These fluctuations are divided into two components based on frequency analysis: HF (0.15–0.40 Hz) and LF (0.04–0.15 Hz). The HF component mainly reflects parasympathetic function and is used as an indicator of parasympathetic stress resilience when seated or at rest. Because the LF component reflects sympathetic and parasympathetic functions, L/H is used as an index of the degree of tension in the sympathetic nervous system, whereas ⊿L/H (increase in L/H compared with sitting and standing) is used as an index of the stress detection power of the sympathetic reflexes (sympathetic switching power). The autonomic activity measured with CVRR is an aggregate of the components of frequency analysis results (HF, LF, etc.) and is used as an index of the sum of autonomic activities. ⊿ CVRR (increase in CVRR compared between sitting and standing) is used as an index of stress reactivity of autonomic nerve activity. Using the measured values of these five items, we calculated the KM score and used it as an evaluation index of total autonomic function.
We evaluated the EAT and bleeding scores at the time of the initial examination and at intervals of approximately 1 month between treatment periods. Changes in each score were analyzed statistically using repeated measures (rm) analysis of variance (ANOVA). Of the 29 patients treated, we evaluated the duration and number of treatments for 24 patients; 5 patients dropped out of the study after the first treatment. The EAT score improved in 13 patients, hemorrhage score improved in 15 patients, and both scores improved in 11 patients. We used one-way ANOVA to compare the following three groups: improvement in EAT score, improvement in bleeding score, and improvement in both EAT and bleeding scores. In addition, we performed multiple comparisons using the Bonferroni method. We evaluated the five autonomic function assessment items and the KM score at the beginning of the EAT treatment and at least 1 month after. A paired t test was used to analyze the results. We performed statistical analyses using the statistical analysis software EZR version 2.6-2. Significance was considered at p < 0.05. We conducted the retrospective observational study, based on existing medical record information, in accordance with the Declaration of Helsinki. All subjects provided verbal and written informed consent. Neither new samples nor data were added to the analysis.
Results
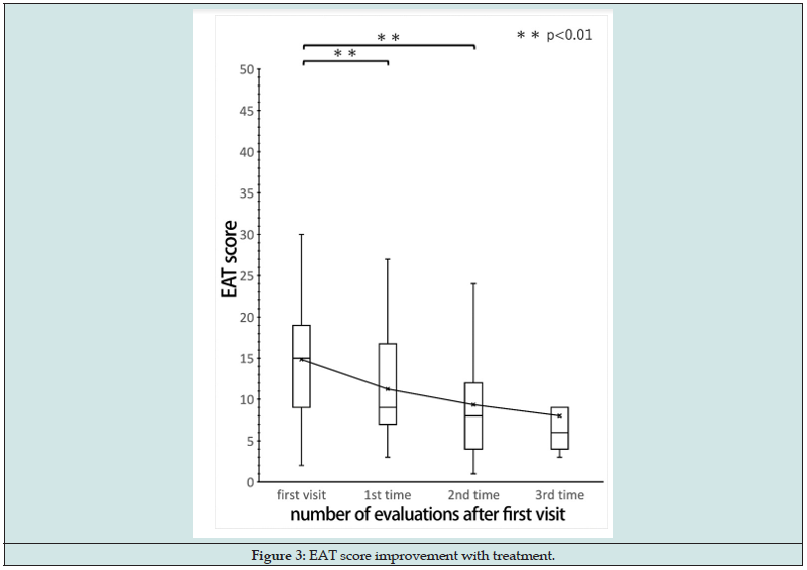
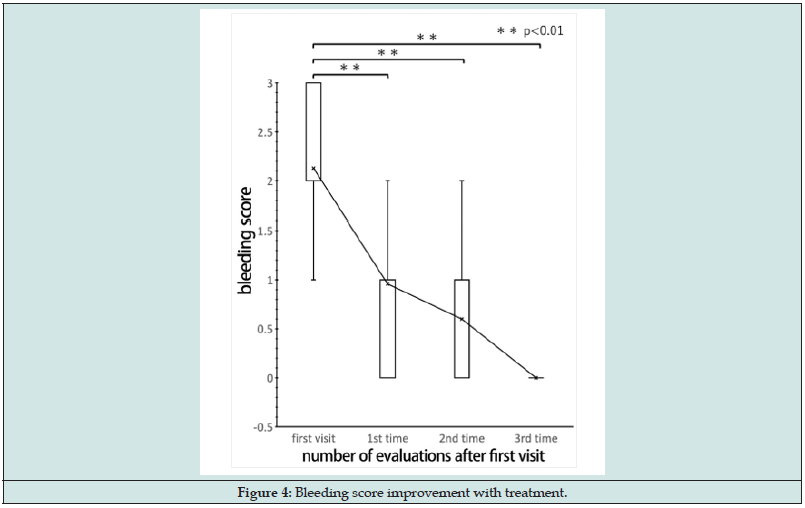
Table 2 presents the main complaints of the 31 LC patients, which were chronic fatigue (n = 13), olfactory dysfunction (n = 7), nasal obstruction (n = 2), posterior rhinorrhea (n = 2), headache (n = 2), sore throat (n = 1), abnormal sensation (n = 1), cough (n = 1), and respiratory distress (n = 1). Of the 31 LC patients who underwent EAT, 29 (93.5%) had chronic epipharyngitis (Table 2). Figure 3 shows the results of EAT scores by rm-ANOVA; we detected significant decreases in the EAT score between the first visit and the first evaluation and between the first visit and the second evaluation (p < 0.01). The mean EAT score, which was 14.9 at the first visit, improved to 11.3 at the second evaluation and to 9.3 at the third evaluation, which is considered as slightly worrisome (Figure 3). Figure 4 displays the results of the rm-ANOVA analysis of the bleeding score. We detected significant decreases in bleeding score between the first visit and the first evaluation, between the first visit and the second evaluation, and between the first visit and the third evaluation (p < 0.01). The mean bleeding score was 2.1 at the first visit and improved to 1.0 at the first evaluation, to 0.6 at the second evaluation, and to almost no bleeding at the third evaluation for the total cases (Figure 4). The trend of improvement in bleeding score tended to be steeper than that of the EAT score.
SD: Standard Deviation
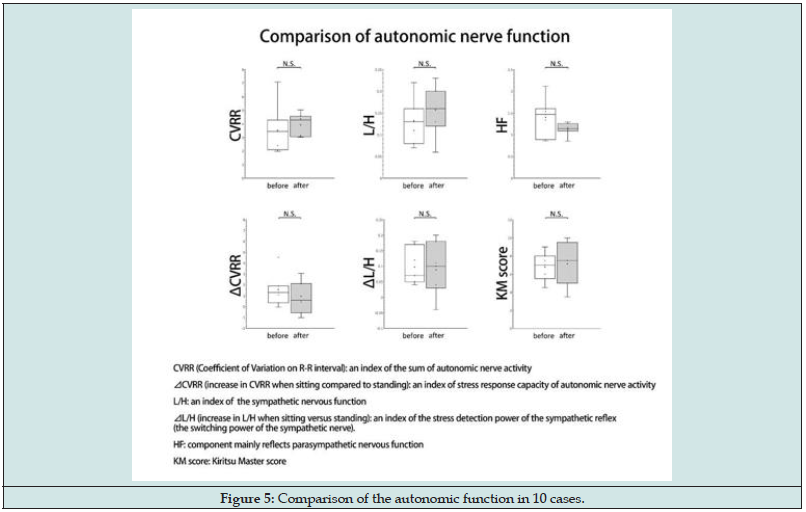
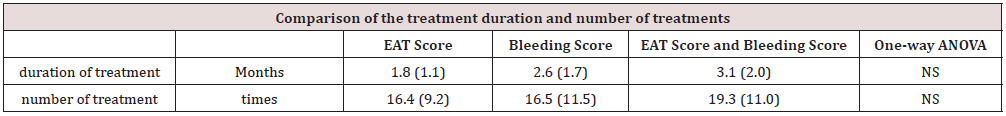
Using one-way ANOVA, we analyzed the duration and number of treatments among the three groups (Table 3) and detected no significant difference; the numbers of treatments required to improve the EAT and the bleeding scores were almost the same (16.4 ± 9.2 to 16.5 ± 11.5). With regard to the duration of treatment, the EAT score improved faster than the bleeding score did (1.8 ± 1.1 to 2.6 ± 1.7). Nevertheless, both scores required more time (3.1 ± 2.0) and frequency (19.3 ± 11.0) to improve. The results suggest that the improvement in subjective symptoms precedes that of objective findings. Of the 29 patients, 10 patients underwent autonomic function tests (Figure 5). The main complaints were chronic fatigue (n= 4), olfactory dysfunction (n= 3), sore throat (n = 1), headache (n = 1), and dyspnea (n = 1). The average duration of treatment until the second examination was 1.5 ± 0.5 months, while the average number of treatments was 11.0 ± 5.6 times. No significant differences were detected at any of the endpoints. However, the sum of autonomic activity CVRR, sympathetic activity L/H, and sympathetic switching power ⊿L/H showed an increasing trend, whereas parasympathetic activity HF and stress reactivity of autonomic activity ⊿CVRR showed a decreasing trend. The KM score increased; the mean score was 5.45 for the first visit, which improved to 5.9 at the second visit. Consequently, autonomic function tends to improve with EAT.
Table 3: Statistical comparison of the three groups. Comparison of the duration and number of treatments in the three groups. The frequency of treatment was almost the same for the improvement in the EAT and bleeding scores. With regard to treatment duration, the EAT score improved earlier. Both scores took time and number of times to improve.
Mean: Standard Deviation; one-way ANOVA: Bonferroni; NS: Not Significant
Discussion
PVFS is considered to be one of the causes of ME/CFS that manifests as symptoms of autonomic dysfunction [6]. Because LC is also considered to be a form of PVFS [1], the prevalence of ME/CFS caused by COVID-19 infection is expected to increase in the future. In this study, we performed EAT on patients with LC who had chronic epipharyngitis, analyzed its therapeutic effect, and discussed its mechanism of action. The examined patients had mean disease duration of 3.5 ± 2.5 months before their visit. The groups of cases included in this study were considered to be primarily infected with the delta strains of COVID, which, as compared with the alpha strains, tend to cause less olfactory and taste disturbance and more symptoms such as fatigue, brain fog, poor concentration, and poor memory [14]. Even after recovery from COVID-19 infection, 61% of patients are reported to have persistent symptoms after an average of 6 months [15]. It is expected that further cases of LC are likely to develop ME/CFS. COVID-19 infects vascular endothelial cells via angiotensin-converting enzyme 2, causing thrombosis and vascular injuries and resulting in dysfunction of various organs [16,17]. The sequelae, such as shortness of breath and dyspnea after pneumonia, are caused by organic damage of the lungs, but various neurologic symptoms are also observed, even in mild cases of COVID-19.
The neuropathic manifestations of LC are thought to be caused by a combination of factors, including direct damage to the cerebral vascular endothelial cells and the possibility of neurodegenerative disease–like changes due to immune dysregulation after infection. In this study, all LC cases were mild with no required hospitalization or other treatment but with high complication rate of chronic epipharyngitis (93.5%), which is thought to induce excretion of cerebral metabolites, resulting in autonomic neuropathy and brain dysfunction [18]. The improvement in LC cases by EAT may be attributed to improvement in the cerebral microcirculation by EAT’s action of scraping and phlebotomy of the pathological mucosa of the nasopharynx and to the improvement in brain function by its autonomic nerve–stimulating action. Ohno reported an overall improvement rate of 86.8% and a 60.3% increase in local findings in patients with common chronic nasopharyngitis after ≥ 10 sessions of EAT once or twice per week [19]. Alternatively, Mogitate et al. reported that EAT performed at least once per week for 12 weeks resulted in improvement in urinary protein and urinary occult blood [20]. Analysis of the treatment courses of EAT and bleeding scores showed a gradual improvement in subjective symptoms but an improvement of the objective findings at an early stage. This results shows that subjective symptoms, such as autonomic and brain dysfunction, require more time to improve, whereas other findings such as nasopharyngeal mucosa and lymphatic congestion by EAT begin to improve in the early stage of treatment. Finally, we found that about 20 treatments over 3 months were required to improve both subjective symptoms and objective findings. Longterm treatment is considered necessary to improve autonomic and brain dysfunction [21].
In the present study, we performed a comparative evaluation of autonomic function after nearly 11 treatment sessions over a period of about 1.5 months, with no significant differences. Comparison between the hemorrhage and EAT scores suggests that the evaluation was performed while the patient had not yet improved in terms of other sensory findings and subjective symptoms. Harada reported that nasopharyngeal stimulation calmed the autonomic symptoms and normalized the vasomotor reflexes [22]. In this study, we observed changes in autonomic function with no significant differences. However, autonomic and sympathetic nerve and reflex activities showed an increasing trend, whereas parasympathetic nerve and autonomic reflex activities showed a suppressing trend. In other words, sympathetic function was improved by EAT, and patients were able to respond normally to stimuli, whereas parasympathetic activity was suppressed with the improvement of chronic epipharyngitis. Using the KM score, which can evaluate overall autonomic function, six patients showed a tendency to improve, whereas four did not. This might be due to the differences in severity of illness and individual differences in autonomic response patterns.
In this study, we did not conduct a comparison with a control group of LC patients who did not receive EAT. However, considering the unclear spontaneous cure rate of LC and that some LC patients continue to have symptoms for more than 6 months with no clear effective treatment for LC, it is highly worthwhile to actively try EAT, which can be performed both safely and noninvasively, for patients with LC who have chronic epipharyngitis. Preventing infections with techniques such as nasal gargling is the most important factor in avoiding LC. Until now, there has been no established procedure to prevent or treat the sequelae of LC, but the results of this study suggest that EAT may be useful for patients with chronic epipharyngitis who have LC. LC, which is associated with medical, social, and economic issues, requires an urgent solution for prevention and treatment. One problem of EAT is the high dropout rate; in this study, 5 of 29 patients (17.2%) stopped coming to the hospital after their first EAT treatment. Based on their estimated age range, many adult patients wanted to return to work as soon as possible, even if they were not completely cured, and treatment and evaluation were therefore incomplete. Although the treatment’s rate of continual improvement is considered to be a further issue, the evaluation method of EAT for LC cases and the duration and frequency of treatment shown in this study may be useful for maintaining and promoting the willingness of LC patients to continue their outpatient treatment.
Conclusions
In this study, we investigated the feasibility of EAT treatment in patients with LC who had chronic epipharyngitis by evaluating a questionnaire, bleeding findings during rubbing, and autonomic function tests. The use of EAT significantly improved the bleeding and interview scores in LC patients with chronic epipharyngitis but did not significantly improve autonomic function tests, which is thought to take more time. EAT is considered to be a useful treatment for patients with LC who have chronic epipharyngitis.
Conclusions
There are no conflicts of interest to disclose in this paper. I would like to express my sincere gratitude to Dr. Manabu Mogitate, Director of Mogitate Otorhinolaryngology, for his guidance on EAT.
References
- Rott R, Herzog S, Fleischer B, Winokur A, Amsterdam J, et al. (1985) Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science 288(4700): 755-756.
- Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, et al. (2021) Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 4(2): e210830.
- Majid F, Ali M, Somayeh M, et al. (2022) Neurobiology of COVID-19. J Alzheimers Dis 76(1): 3-19.
- Undine-Sophie D, Angelica V, Valentina F, et al. (2021) Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): An Overview. J Clin Med 10(20): 4786.
- Peter L, Toogoodab D, Clauwc S (2021) Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Where will the drugs come from? Pharmacological Research 165: 105465.
- Komaroff AL, Takahashi R, Yamamura T, Sawamura M (2018) Neurologic abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: a review. Brain Nerve 70(1): 41-54.
- Horiguchi S (1975) The discovery of the nasopharyngitis and its influence on general diseases. Acta Otolaryngol 329: 1-120.
- Tanaka A, Hotta O (2022) The efficacy of intranasal sphenopalatine ganglion stimulation (INSPGS) in long COVID, and its possible mechanisms. Sch J Oto 8(2): 860-864.
- Imai K, Yamano T, Nishi S, Nishi R, Nishi T, et al. (2022) Epipharyngeal abrasive therapy (EAT) has potential as a novel method for Long COVID treatment. Viruses 14(5): 907.
- Tanaka A, Hotta O, Nagano C (2021) Efficacy of epipharyngeal abrasive therapy (EAT) in myalgic encephalomyelitis/chronic fatigue syndrome (MC/CFS) and hypothesis of action mechanism. J Jpn Fatigue Soc 15(2): 18-26.
- Tanaka A (2018) Studies on band-limited light endoscopic diagnosis and endoscopic epipharyngeal abrasive therapy in chronic epipharyngitis. Stomato-pharyngeal 31(1): 57-67.
- Spotnitz, William D, Zielske, Dirk, Centis, Valerie (2018) The SPOT GRADE; A New Method for Reproducibly Quantifying Surgical Wound Bleeding. SPINE 1(43): 664–671.
- Okada N (2015) Autonomic nerve function test 5th Japanese Society of Autonomic Neurology. Bunkodo, Tokyo, Japn pp. 183-198.
- Vinod Nikhra (2021) COVID-19 and Long Covid: Organs Damage and Dysfunctions, and Implications for Clinical Course. Heighten Science Publications Corporation, Texas, USA.
- Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, et al. (2021) Long COVID in a prospective cohort of home-isolated patients. Nat Med 27(9): 1607-1613.
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, et al. (2020) Endothelial cell infection and endothelitis in COVID-19. Lancet 395(10234): 1417-1418.
- Nishi k, Yoshimoto S, Nishi S, Tsunoda T, Ohno J, et al. (2022) Epipharyngeal abrasive therapy down-regulates the expression of SARS- CoV-2 entry factors ACE2 and TMPRSS2. In Vivo 36(1): 371-374.
- Hotta O, Nagano C, Tanaka A, Ieiri N (2017) Possible mechanisms underlying epipharyngeal abrasive therapy (EAT) with ZnCl2 solution for the treatment of autoimmune diseases and functional somatic syndrome. J Antivir Antiretrovir 9(4): 81-86.
- Ohno Y (2019) Effectiveness of epipharyngeal abrasive therapy for chronic epipharyngitis. Stomato-Pharyngology 32(19): 33-39.
- Mogitate M, Sasaki Y, Komiyama A (2021) Outcome of an outpatient specialty clinic for chronic epipharyngitis. Auris Nasus Larynx 48(3): 451-456.
- Giandomenico B, Fabrizio R, Vittoria R (2021) Post-Acute Sequelae of COVID-19 and Cardiovascular Autonomic Dysfunction: What Do We Know? J Cardiovasc Dev Dis 8(11): 156.
- Harada S (1967) Relation between autonomic nerve symptoms and finger vasomotor reflex in epipharyngitis. Nihon Jibiinkoka Gakkai Kaiho 70(4): 841-856.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...