Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Case Report(ISSN: 2641-1709) 
Cochlear Implantation in Mondini Malformation Using a Slim Modiolar Electrode Volume 8 - Issue 2
Mariapaola Guidi* and Franco Trabalzini
- Department of Otorhinolaryngology, Meyer Children’s Hospital, Italy
Received: April 21, 2022; Published: May 03, 2022
Corresponding author: Mariapaola Guidi, Department of Otorhinolaryngology, Meyer Children’s Hospital viale Pierraccini 24, 50139 Florence, Italy
DOI: 10.32474/SJO.2022.08.000284
Abstract
Background: Mondini malformation is a birth defect with a shortened inner ear with only one and a half cochlear turns which can be treated with cochlear implant (CI). A new generation of modiolar electrodes are designed to precisely fit in the curved space of the cochlea.
Case Presentation: A 21-month-old boy affected by Mondini malformation and congenital bilateral profound hearing loss underwent to CI surgery and fitted with a curved modiolar electrode.
Conclusions: Pre, intra and post-operative impedance measurements were taken with a Cochlear™ Nucleus® CI 532 electrode. Progress was satisfactory and even exceeding the many similar reported cases of straight electrode fittings.
Keywords: Mondini; cochlear implant; modiolar electrode; straight electrode; hearing loss
Abbreviations: CI: Cochlear Implant; HL: Hearing Loss; IEM: Inner Ear Malformations; IP: Incomplete Partition; ABR: Auditory Brainstem Responses; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; NRT: Neural Response Telemetry; ECAP: Evoked Compound Action Potentials; CNC: Consonant-Nucleus-Consonant; PM: Peri-Modiolar; LW: Lateral Wall; PTA: Pure Tone Audiometry; CAP: Category of Auditory Perception; CH: Cochlear Height
Introduction
Congenital hearing loss (HL) is the most common birth defect, estimated to affect 2-3 in every 1000 births. About 20% of patients affected by congenital sensorineural HL have inner ear malformations (IEM) that are detectable by radiology. These IEM are bilateral in 50% of cases [1]. In the IEM classification system, only Michel deformity, rudimentary otocyst and cochlear aplasia cannot be subjected to CI surgery. All other IEM such as cochlear aplasia, common cavity, cochlear hypoplasia, incomplete cochlear partition (IP) type I-II-III, enlarged vestibular aqueduct and cochlear aperture abnormalities, can be surgically treated with CI [2]. The neuronal populations range is 7677 in an ear with Mondini while the norm for human subjects is 35 000 neurons. The length of the spiral ganglion varies from 7.3 mm to 14.8 mm, the norm for human subjects is 12 mm [2,3]. In literature is reported that the minimum number of ganglion cells stimulated by the cochlear implant would be 5000 [4]. Many implantable electrode array designs have been proposed with different lengths and angular insertion depths, according to the patient cochlea’s size, shape, anatomy and pathology [3]. The initial designs were all straight, but a new generation of electrodes are curved or modiolar, designed to fit close to the modiolus, or curved inner axis of the cochlea [3,5]. In this paper we evaluate a clinical case regarding a child affected by congenital bilateral sensorineural HL with incomplete cochlear partition type II (Mondini malformation) who underwent CI surgery using a slim modiolar electrode.
Clinical Case
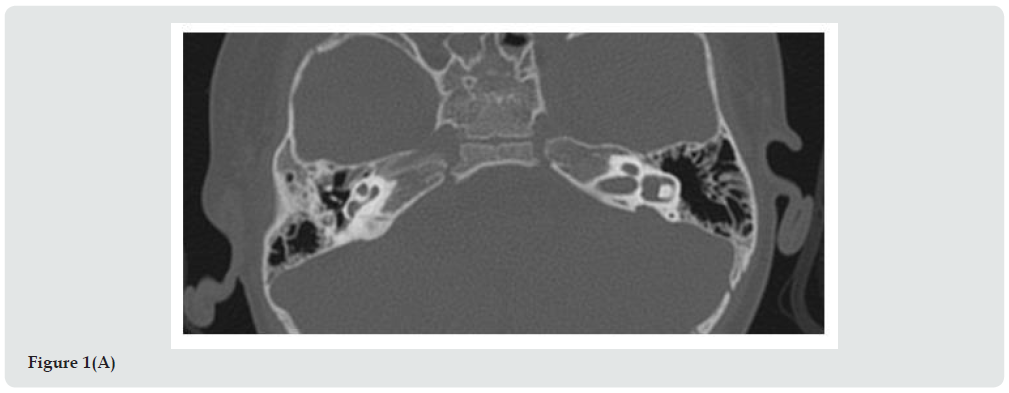
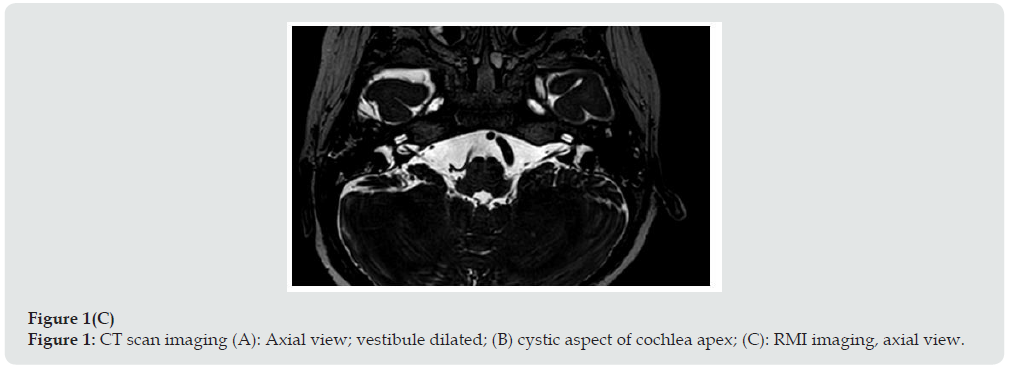
Between 2016 and 2019 seventy-five CI procedures in children were performed in our Department of Otorhinolaryngolgy. Thirty- seven patients of these presented IEM. Seven patients were affected by Mondini malformation. A 21-month-old boy was admitted to our ENT department. He was born at 38 weeks gestation with a birthweight of 2.8 Kg. Three days later jaundice had appeared, and the child underwent phototherapy. Cardiological evaluation was regular, and the urine test revealed no Cytomegalovirus. Newborn’s hearing screening, performed by automated transient otoacoustic emissions and Automated Auditory Brainstem Response, was worrying with a recommendation of bilaterally “refer”. When we evaluated the infant for the first time the hearing threshold potentials evoked from the brainstem (Auditory Brainstem Responses, ABR) had already been performed at another hospital with the diagnosis of severe deafness on the left side and moderatesevere deafness on the right side. The patient thus used bilateral hearing aids since 5 months of life. He started babbling at 10 months, but then speech development completely stopped. He underwent neuropsychological evaluation and was given speech therapy regularly three times a week. Genetic evaluation was performed, and the analysis is still ongoing. At 21 months we repeated the ABR, and the V wave threshold was 95 dB hearing loss on the right side and 100 dB on the left, at 2000-4000 Hz. Conditioned behavioral audiometry put threshold at greater than 100 dB. With hearing aids, the threshold was nevertheless reduced to 60 dB. Brain magnetic resonance imaging (MRI) under sedation revealed dilated vestibule and enlarged vestibular aqueduct bilaterally. Both cochleas were dysmorphic in appearance. The VII and VIII cranial nerves appeared normal. Computed tomography (CT) scan imaging confirmed the pathological aspect of the cochlea, in fact the apex had a cystic appearance, the vestibule was dilated, and the vestibular aqueduct was enlarged (Figure 1). Considering the bilateral profound hearing loss, CI surgery was proposed to the parents who accepted. Simultaneous bilateral CI was performed when the child was 24 months old.
Figure 1C: CT scan imaging (A): Axial view; vestibule dilated; (B) cystic aspect of cochlea apex; (C): RMI imaging, axial view.
Surgical Procedure
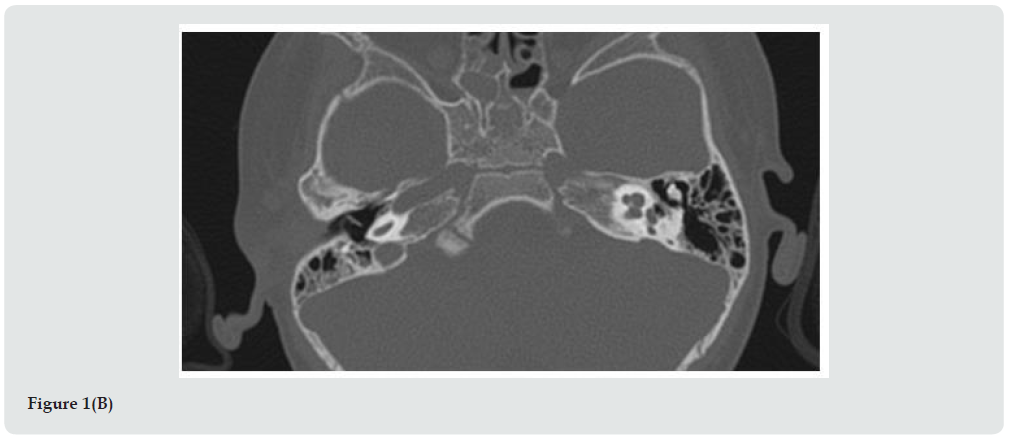
The posterior tympanotomy was extended inferiorly and a promontorial cochleostomy was performed because the round window niche appeared narrowed. The CI used was a Cochlear™ Nucleus® CI 532. The insertion of the electrode into the cochlea was complete without any oozing phenomenon. The cochleostomy was closed using a piece of temporal muscle. Intraoperative threshold nerve response, Automatic Neural Response Telemetry (NRT), were low as shown in Figure 2. This test represents an important intraoperative assessment of audiological response of the auditory nerve, of its integrity, a good electrode proximity to the modiolus and, thus, its correct cochlear insertion [6]. ECAP test (Evoked Compound Action Potentials) is based on multiple stimuli given by a certain electrode of cochlear implant array at increasing levels of energy with a consequent depolarization of auditory nerve fibers, recorded by electrodes near to the stimulating one. ECAP provides information regarding the status of the auditory nerve and, intraoperatively, confirms also the device integrity and electrode functionality. In our case, ECAP test was performed on all 22 electrodes of the array and Figure 2 reports the minimum level of energy used to induce a neural depolarization; in this figure x-axis represents the number of each tested electrode and y-axis represents level of energy current. In literature it is known that lower NRT curves are associated to a better auditory nerve functionality, a closer distance to the stimulation target (modiolus) and a reduced period of hearing deprivation. Moreover, lower responses mean a more selective recruitment of nerve fibers. In the post-operative fitting low NRT levels allow less energy levels of map profiles, increasing the autonomy of speech processor batteries. According to some authors, generally NRT curves show a horizontal trend along the implant array or at least a homogeneous trend between close electrodes. In our case, the patient was affected by a cochlear malformation and the small variations on electrodes 6, 13, 15 and 19 could be explained by that [7].
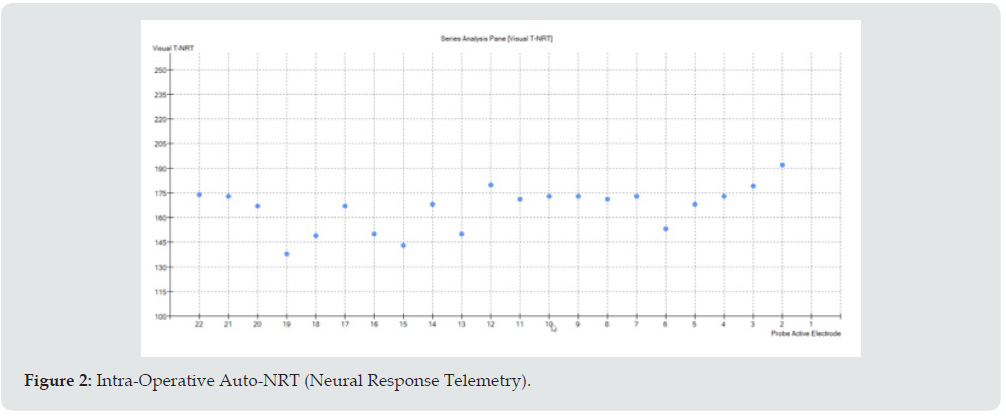
Intraoperative electrical impedances of all 22 electrodes are reported in Figure 3a. Impedance test is an intraoperative and postoperative measurement which shows the correct functioning of each electrode of the array. Impedance is a value expressed in Ohm, thus it corresponds to the resistance offered to the current flow emitted from stimulating electrode through endocochlear perilymph to the recording electrode. Impedance value is also correlated to the possible presence of other material that could be found in the interface between platinum electrode and perilymph, such as blood or air bubbles. Each implant has its own typical range of impedance values, ranging from few kOhms up to a maximum value of 25 kOhms. If impedance value tends to zero, a short circuit has to be verified since it could represent a failure of the measured electrode (i.e., a defect of insulating material of the array or a damage during insertion); if impedance value exceeds the maximum value, an open circuit could be present, and it could depend on a breakage of the conductor wire or an insulating layer (air).
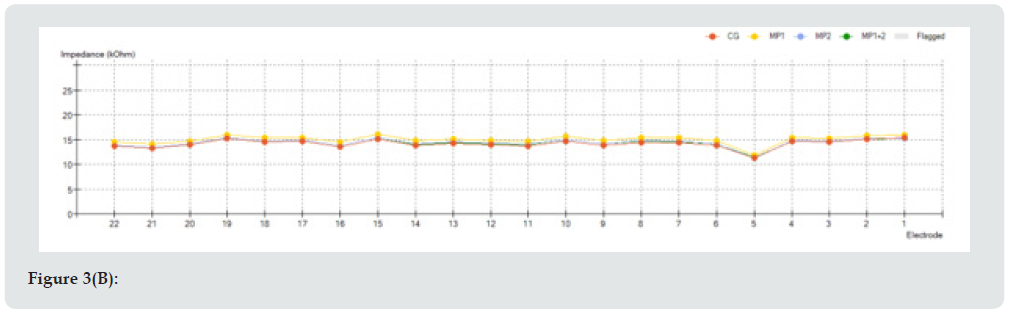
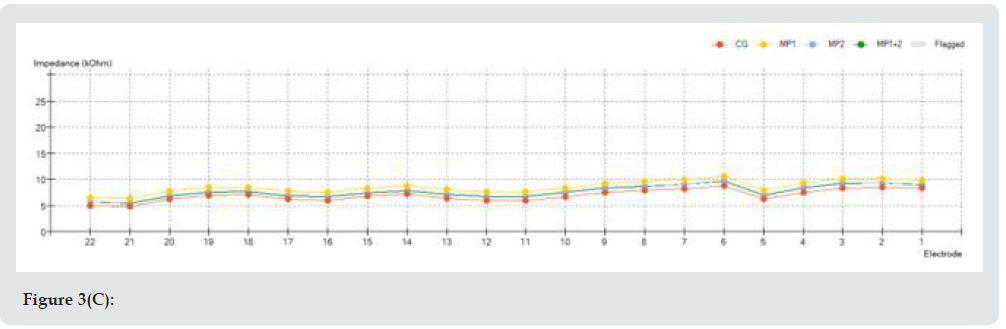
Figure 3D: (A): Intra-operative impedance measurements; (B): First fitting impedance measurements one month after surgery, (c): Six months fitting impedance measurements; (D): One year fitting impedance measurements.
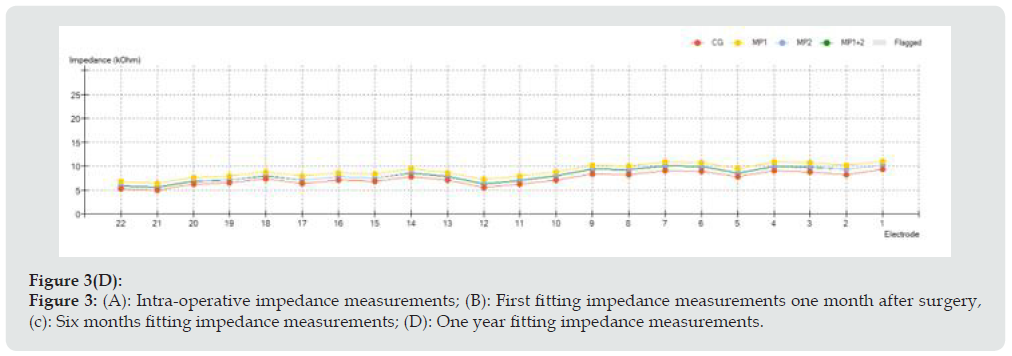
Nevertheless, impedance test does not confirm itself a correct electrode positioning into tympanic scale. For this reason, other tests are required, such as NRT or spread of excitation. Curve trend in impedance test has to be checked in order to verify that a sawtooth profile is not revealed; a smooth curve is generally the curve we aim to obtain. In our case, a smooth pattern was observed both during intraoperative and postoperative impedance measurements, with a value ranging from 5 to 20 kOhms intraoperatively; from 5 to 10 kOhms 6 and 12 months after surgery. It is important to remember that a low value of impedance indicates a closer contact between electrode and endocochlear fluid, but it requires an increased amount of energy for fitting [8]. The switch-on and the first CI programming were performed 3 weeks after surgery. First programming consisted of 4 listening programs, called maps, each with increased levels of stimulation current based on AutoNRT response performed on the day of switch-on. Postoperative implant impedance measurements were taken at 3 weeks, 6 months and one year after surgery and are reported in Figure 3.
Discussion
In CI surgery, the aim is to place all of the stimulating contacts inside of the cochlea without causing any degree of intra-cochlear damage [5,6]. It is known in literature that spiral ganglion cells are the main neural elements electrically stimulated and the number of spiral ganglion cells required for electrical stimulation is much less than that required for acoustic stimulation [9-11]. This may explain why auditory and speech outcomes could be not significantly different between children with and without Mondini malformation [12-14]. Suri et al. showed that after implant use for one year, both children with radiologically normal inner ears and children with Mondini dysplasia had similar speech perception scores.8 The study performed by Chen et al in 2014 showed that the auditory skills of young children with Mondini dysplasia developed rapidly over a period of 3 years after cochlear implantation, in a similar manner to those of young children with radiologically normal inner ears [15].
Sturm affirmed that in children with normal ears Consonant- Nucleus-Consonant (CNC) scores increased over time after implantation across all array designs. Perimodiolar (PM) array had higher CNC scores compared to lateral wall (LW) electrodes, particularly 6-months after CI. No significant differences were observed after 12 months in mean pure tone audiometry (PTA) and speech recognition, but a faster acquisition of auditory results was observed in patients treated with CI532 [16,17]. Straight LW electrodes stimulate the nerve fiber endings of the auditory neurons at the organ of Corti whereas PM electrode arrays, which are closer to the modiolar wall, stimulate the spiral ganglion cells. In fact, LW electrode is near to the basilar membrane while the PM electrode has much greater clearance in terms of the medial scala tympani height and much greater proximity to the spiral ganglion, as well as being much further from the functional structures of the basilar membrane and the organ of Corti [6,7,11,17]. Schwartz showed that the type of array was not associated with better word recognition outcomes in long-term follow-up in patients with IP type II [18,19]. The full insertion of electrodes would be required to bring the stimulation contacts closer to the modiolus wall. Frijns et al found no correlation between the distance to the modiolus and the threshold or the maximum levels with a straight lateral wall electrode [20,21,22].
In the study performed by Grover et al. in 2017, five patients out of seventeen with EAM, presented Mondini malformation. The audiological results were not affected by the presence of cochleovestibular anomalies, and the straight array device was used in all patients [23]. The PM electrodes have a pre-determined shape which may not match the coiling pattern of individual cochlear geometries; moreover, they may more likely cause trauma than other electrodes because of the stylet wire which brings the electrode to be in a straight configuration before complete insertion occurs [24,25]. The length of the electrode in IP is important to avoid the electrode entering into the internal auditory canal with the risk risk of an intraoperative cerebrospinal fluid gusher/oozing [9,23,25]. Typically, the length of the electrode should match the length of the basal turn/middle turn of the cochlea. In case of cerebrospinal fluid gusher in malformed cochlea, a cork type plug at the electrode array stopper point could provide a solution [26]. In our clinical report the insertion of the PM electrode into the cochlea was complete, there was no oozing phenomenon, and no other complications were reported during or after surgery. Six months after CI surgery, the mean PTA with both implants was 45 dB HL while one year after surgery the mean PTA was 30 dB HL. In according to literature, CI532 electrode reduce the distance to the modiolus with better audiological performances and more focused stimulation [27]. In our case, Category of Auditory Perception and Speech Intelligibility Rating were difficult to evaluate due to the language difficult. In fact, both parents don’t speak Italian and the child responds to commands only if formulated in Chinese.
Conclusion
In the clinical report presented, the PM slim electrode array has been used and its insertion into the cochlea was complete, without oozing phenomenon and no other complications during or after surgery. One year after surgery the mean PTA was 30 dB HL.
Acknowledgements
The authors acknowledge the Cochlear employees Filippo Morini and Paolo Malerba, which contributed to the presented work providing the model for measurement and analysis of neural response telemetry.
Authors’ contributions
Mariapaola Guidi: Manuscript writing, imaging formatting, clinical analysis. Franco Trabalzini: Content and clinical analysis.
Funding
The authors declare that there are no financial disclosures involved in the manuscript.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- Sennaroglu L, Bajin MD (2017) Classification and current management of inner ear malformations. Balkan Med J 34(5): 397-411.
- Ozkan HB, Cicek Cinar B, Yucel E, Sennaroglu G, Sennaroglu L (2021) Audiological performance in children with inner ear malformations before and after cochlear implantation: a cohort study of 274 patients. Clin Otolaryngol 46(1): 154-160.
- Schmidt JM (1985) Cochlear neuronal populations in developmental defects of the inner ear. Implications for cochlear implantion. Acta Otolaryngol 99(1-2): 14-20.
- Paparella MM, el-Fiky FM (1972) Mondini’s deafness. Arch Otolaryngol 95: 134–140.
- Sun JQ, Sun JW, Hou XY (2017) Cochlear implantation in Mondini’s deformity: could the straight electrode array with length of 31 mm be fully inserted? Acta Otolaryngol 137(7): 712-715.
- Mittmann P, Todt I, Ernst A (2016) Electrophysiological detection of scalar changing perimodiolar cochlear electrode arrays: a long term follow-up study. Eyr Arch Otorhinolaryngol 273(12): 4251-4256.
- Mewwns A, Brademann G, Hey M (2020) Comparison of perimodiolar electrodes: imaging and electrophysiological outcomes. Otol Neurotol 41(7): e934-e944.
- Shaul C, Bester CW, Weder S (2019) Electrical impedance as a biomarker for inner ear pathology following lateral wall and peri-modiolar cochlear implantation. Otol Neurotol 40(5): e518-e526.
- Miyamoto RT, Robbins AJ, Myres WA, Pope ML (1986) Cochlear implantation in the Mondini inner ear malformation. Am J Otol 7(4): 258-261.
- Silverstein H, Smouha E, Morgan N (1988) Multichannel cochlear implantation in a patient with bilateral Mondini deformities. Am J Otol 9(6): 451-455.
- Dhanasingh A, Hochmair I (2021) Special electrodes for demanding cochlear conditions. Acta Otolaryngol 141(sup1): 157-177.
- Kumari A, Arumugam SV, Malik V, Goyal S, Kameswaran MJ (2021) Audiological and Surgical Outcomes of Pediatric Cochlear Implantation in Mondini's Dysplasia: Our Experience. Int Adv Otol 17(1): 19-22.
- Demir B, Cesur S, Sahin A, Binnetoglu A, Ciprut A, et al. (2019) Outcomes of cochlear implantation in children with inner ear malformations. Eur Arch Otorhinolaryngol 276(9): 2397-2403.
- Suri NM, Prasad AR, Sayani RK, Anand A, Jaychandran GJ (2021) Cochlear implantation in children with Mondini dysplasia: our experience. Laryngol Otol 135(2): 125-129.
- Chen X, Yan F, Liu B (2014) The development of auditory skills in young children with Mondini dysplasia after cochlear implantation Plos One 9(9): e108079.
- Sturm JJ, Patel V, Dibelius G, Kuhlmey M, Kim AH (2021) Comparative performance of lateral wall and perimodiolar cochlear implant arrays. Otol Neurotol 42(4): 532-539.
- Garaycochea O, Manrique-Huarte R, lazaro C (2020) Comparative study of two different perimodiolar and a straight cochlear implant electrode array: surgical and audiological outcomes. Eur Arch Otorhinolaryngol 277(1): 69-76.
- Schwartz N, Brown KD, Park LP (2020) Audiologic outcomes of cochlear implantation in cochlear malformations: a comparative analysis of lateral wall and perimodiolar electrode arrays. Otol Neurol 41(10): e1201-e1206.
- Wang B, Cao KL, Wang Y , Li H (2017) Comparison of hearing rehabilitation for patients with Mondini malformation after cochlear implantation with different electrodes. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 31(4): 284-289.
- Esquia Medina G, Borel S, Nguven Y, Ambert-Dahan E, Ferrary Evelyne, et al. (2013) Is electrode-modiolus distance a prognostic factor for hearing performances after cochlear impant surgery? Audiol Neurootol 18(6): 406-413.
- De Seta D, Mancini P, Russo FY, Torres R, Mosnier I, et al. (2016) 3D curved multiplanar cone beam CT reconstruction for intracochlear position assessment of straight electrodes array. A temporal bone and clinical study. Acta Otorhinolaryngol Ital 36(6): 499-505.
- Van der Beek F, Briaire JJ, Frijins JHM (2015) Population-based prediction of fitting levels for individual cochlear implant recipients. Audiol Neurootol 20(1): 1-16.
- Grover M, Sharma S, Bhargava S, Singh SN, Gupta G, et al. (2017) Cochlear implantation in children with anomalous cochleovestibular anatomy: our experience. Indian J Otolaryngol Head Neck Surg 69(4): 504-508.
- Martinez-Monedero R, Niparko JK, Aygun N (2011) Cochlear coiling pattern and orientation differences in cochlear implant candidates. Otol Neurotol 32(7): 1086-1093.
- Breinbauer HA, Praetorius M (2015) Variability of an ideal insertion vector for cochlear implantation. Otol Neurotol 36(4): 610-617.
- Sennaroğlu L, Atay G, Bajin MD (2014) A new cochlear implant electrode with a “cork”-type stopper for inner ear malformations. Auris Nasus Laynx 41(4): 331-336.
- Holden LK, Firszt JB, Reeder RM, Uchansku RM, Dwyer NY, et al. (2016) Factors affecting outcomes in cochlear implant recipients implanted with a perimodiolar electrode array located in scala tympani. Otol Neurotol 37(10): 1662-1668.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...