Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Review Article(ISSN: 2641-1709) 
Classification, Pathophysiology, Genetics, And Role of Lifestyle Medicine in Presbycusis Volume 6 - Issue 3
Adaobi Elizabeth Osuji*
- Ear, Nose and Throat Department, University of Port Harcourt Teaching Hospital, Nigeria
Received: April 26, 2021 Published: May 06, 2021
Corresponding author: Adaobi Elizabeth Osuji, Ear, Nose and Throat Department, University of Port Harcourt Teaching Hospital, Port Harcourt, Rivers State, Nigeria
DOI: 10.32474/SJO.2021.06.000238
Abstract
Presbycusis, which is age-related hearing loss is hearing loss usually seen in the elderly due to advancing in age, marked by a higher hearing threshold usually worse at higher frequencies, which impairs speech discrimination in noise. Genetic predisposition is an important factor in the aetiology of presbycusis. Presbycusis was previously classified into 4 different types, and recently into 6 types with the addition of mixed, and indeterminate types of presbycusis, with each type reflecting a peculiar audiometric configuration and speech discrimination percentage. Artificial intelligence has utilized this peculiar audiometric feature in diagnosis of genetic hearing loss. and recently, lifestyle medicine is being employed to highlight the benefit of preventive medicine in the management of hearing loss. Human studies have remained the trend of genetic hearing loss research in recent years. There is an overwhelming interest, with reasonable insight gained into the pathophysiology and genetics of ARHL, but the interaction of genetics, lifestyle, and environment certainly complicates our ability to separate their individual contributions to this pathology. There is need to improve research in the role of lifestyle medicine in curbing presbycusis.
Keywords: Presbycusis; age-related hearing loss; audiometry; genetics; lifestyle medicine
Introduction
Presbycusis or age-related hearing loss is a progressive bilateral symmetrical sensorineural hearing loss seen in people with advancing age, which is more pronounced at the higher frequencies. It is a complex multifactorial disorder characterized by a negative shift in audiometric threshold, with a decline in speech understanding and speech perception, especially in noisy environment [1]. As a result of these, there is an impaired physical, cognitive, social, and emotional functioning of the individual with a resultant poor quality of life. Presbycusis can also lead to depression, social isolation, and a lowered self-esteem [1]. The prevalence of age-related hearing loss has been estimated in several parts of the world using audiometric studies [2]. The use of different audiometric criteria has resulted in varied prevalence estimation in same location and in different parts of the world. For example, to estimate prevalence of age-related hearing loss in the US population, the Framingham study estimated 29% in subjects older than 60 years, the Beaver Dam project estimated 73% in subjects older than 70 years, and the Health ABC study estimated 60% in subjects 73-84 years, whereas using the WHO criteria, the prevalence of age-related hearing loss in the population is 63.1% [3]. These epidemiological studies have also provided an in-depth understanding of the modifiable and non-modifiable risk factors of age-related hearing loss [3,4]. Being considered multifactorial, several underlying factors can be involved in the promotion of this disorder [4]. These factors can be considered non-modifiable like the biological (age, ethnic origin, gender) and genetic predisposition of the individual [3,5]. It can also have contributions from modifiable factors like the lifestyle (smoking, drinking, diet), systemic diseases as co-morbidities (diabetes mellitus, hypertension) or environmental (ototoxic medication, exposure to noise). In addition, some processes in the body related to aging which also affect hearing include oxidative stress and mitochondrial DNA damage which is worsened by reduction in blood supply to the cochlea and ionic dysregulation because of end cochlear potential loss [4]. Among the list of nonmodifiable risk factors, age and male gender has a strong positive association with hearing loss [2]. As much as the black race has been reported to be a protective factor, genetic phenotype has been found to be a substantial non-modifiable risk factor, and heritability longitudinal studies in families have shown a heritability index of 0.35-0.55 [3].
Classification of Age-Related Hearing Loss
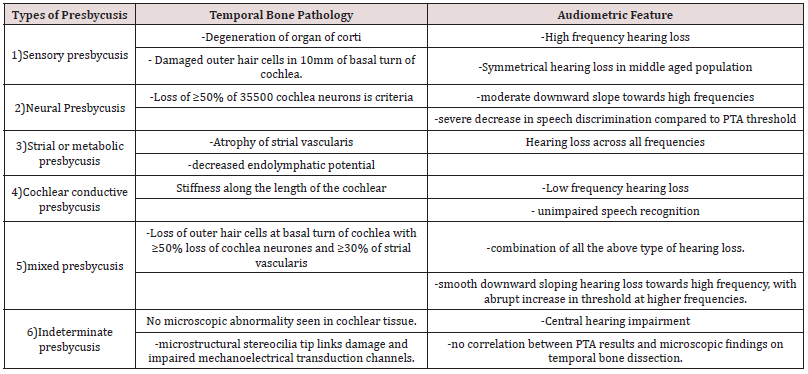
Schuknecht in 1969 classified presbycusis into four groups, with due consideration to temporal bone pathology and hearing test results. He identified the four types as Sensory, Neural, Metabolic or Strial, and Cochlear conductive presbycusis [1]. In 1993, Gacek added Mixed and Indeterminate types, to the existing four, which made six categories or types of presbycusis [1]. However, Dawes and Payton, in 2016 stated that there are three types of presbycusis or age-related hearing loss, viz: Sensory presbycusis, Strial presbycusis or Metabolic presbycusis, and Neural presbycusis. They further stated that most patients will have a combination of two or more of the various types of presbycusis. The table below highlights the histologic and audiometric features of types of presbycusis according to Schuknecht and Gacek classification (Table 1).
Pathophysiology of Age-Related Hearing Loss
As much as the age of onset and the rate of progression varies, the cochlea is central in the pathophysiology of presbycusis and there are obvious signs of cochlear degeneration present in persons with presbycusis [6]. Tu and Friedman in 2017 reported that the atrophy of the stria vascularis, decreased Na+ K+ ATPase function and its resultant effect on endolymphatic potential has been found to be paramount in the pathology of the aging cochlea. The Schuknecht classification of presbycusis based on histopathologic changes have been criticized as confusing and inadequate, because it did not consider role of other cells in the cochlea apart from outer hair cells in its pathology of age-related hearing loss. The changes in the central auditory system could also be implicated in the pathophysiology of presbycusis [1]. Moreover, atrophy or rupture of the Reisner’s membrane which can occur due to ageing can also be the cause of presbycusis [7]. The changes in the central auditory system that can contribute to age related hearing loss are shown below:
Changes in neurotransmitters and voltage-gated channels
Changes in the K-channel subunits Kv1.1 and Kv1.3 were noted in the cochlear neuron due to aging, as well as changes in hyperpolarization due to glycine-evoked changes [8]. These glycine-evoked changes caused the activation of the K-channel subunits of the potassium channel to be decreased. This was reported by Jung et al in their study titled age-related changes in the distribution of Kv1.1 and Kv1.3 in rat cochlear nuclei [8]. It has also been reported that calcium binding proteins like parvalbumin, calbindin, and calretinin increase with age in cochlear nucleus and lead to degeneration of the cochlea [9]. The changes with calcium binding proteins are such that the number of the nerve fibres that are calcium-binding protein specific increased proportionately during aging, in a protein-specific and region-specific behaviour [9]. Variations due to aging were also observed with glutamate neurotransmitter receptors during depolarization on auditory neurons. In the study done with Gerbils, the activity of glycine and GABA neurotransmitters reduced with increasing age, and an increase in nitric acid was also observed [8]. Loss of GABA associated neurons have been observed in age-related hearing loss [1].
Changes in central auditory system
Age related changes also occur in the Central auditory centres, and results more from deterioration in the function of neurotransmitters and synapses, rather than from neuronal loss [6]. Glial fibrillar protein, whose activity helps to maintain cochlear function, has been found to reduce during the process of aging in mice, and about 20% loss of nerve fibres was seen in the vestibulocochlear nerve, especially in the superior olivary complex [7]. These changes lead to a reduced coding intensity, increasing the deficit in temporal processing, resulting in low speech understanding especially in background noise [1]. This has been described as central auditory processing disorder (CAPD), and usually occurs in subjects aged 65 years and above [3]. In this elderly population >65 years, a prevalence of CAPD ranging from 9% to 14% has been reported, and early diagnosis and treatment of presbycusis has been shown to prevent or reduce cognitive impairment in the elderly [3]. However, this is an aspect of presbycusis that is open to further research. In addition to the afore-mentioned changes in the central nervous system, the effect of oxidative stress from reactive oxygen species causes age related changes in the central auditory system, antioxidant enzymes in glutathione metabolism, catalase and methionine sulfoxide reductase have all been found in the aging cochlea [1,7]. Oxidative brain stress can lead to age related reduction in cognition, concentration and memory which contribute to the features of presbycusis [6].
Cochlear synaptopathy
In the study done by Kujawa and Liberman in animal model using immunostaining, they reported loss of cochlear synapse, and a delayed spiral ganglion cell degeneration without any evidence of loss of hair cell [10]. This is termed cochlear synaptopathy. While the mechanism of this injury has not been fully understood, glutamate toxicity resulting in swelling of nerve terminals have been implicated [7]. GRM7 a gene-encoding glutamate metabotropic receptor 7 which is expressed in hair calls and spiral ganglion neurones, have been strongly implicated in ARHL pathogenesis [5]. Its activation normally reduces release of glutamate, however, a variant or mutant form of GRM7 can alter susceptibility and lead to increased glutamate release to toxic levels within the synapse leading to cochlear synaptopathy [5]. Cochlea synaptopathy has been suggested to be a primary pathology in both noise-induced hearing loss (NIHL) and age-related hearing loss (ARHL) [5]. Spiral ganglion cells have been reported to decrease with age, despite normal hair cell population, and preferentially affects auditory nerve fibres with low spontaneous discharge rate Kujawa and Liberman [10]. This can be responsible for reduced speech to noise ratio experienced by people with age related hearing loss [7,10].
Audiometry and Age-Related Hearing Loss
Figure 1: Audio gramatic representation and speech discrimination of types of presbycusis according to Schuknecht and Gacek classification. A=sensory, B=Neural, C=Strial, D=Cochlear, E=Indeterminate, F=Mixed..
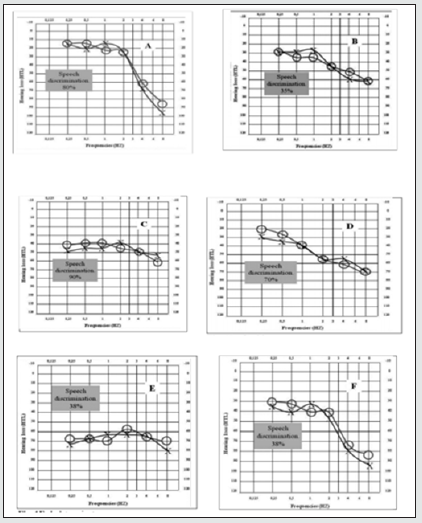
Pure tone audiometry and speech recognition tests have also been applied in the study of age-related hearing loss. Gates et al in their study analysing the shape of audiograms in age related hearing loss, reported that heritability was greater in strial presbycusis than for sensory hearing loss [11]. Greater heritability has been reported for a flat audiometric shape characteristic of overall severity across all frequencies, which is consistent with strial hearing loss, and a lower heritability for steep sloping high frequency hearing loss which is characteristic of sensory loss [4]. Higher estimates of heritability have been obtained for audiometric as against speech recognition-based phenotypes, which suggest that although audiometric and speech recognition-based tests may overlap, they could provide complementary information about the contribution of genetics to different underlying pathologies of ARHL [12]. Following the Schuknecht and Gacek classification of Presbycusis into 6 types [2], their various audiometric configuration with speech discrimination pattern has been described as shown in the diagrams in Figure 1 below. As much as age related hearing loss can be detected with characteristic configuration on speech and audiometric assessment, there is no clinical method to predicts its onset in advance and there is no established medical prevention nor treatment that can be employed to restore or reverse hearing loss after its onset [1].
Genetics of Age-Related Hearing Loss
It has been shown that genetic predisposition is a huge risk factor for ARHL, with significant heritability indices already reported, thus there have been efforts to research on the genetics of the pathology of ARHL in humans. Lewis et al. in 2018 [13] stated that heritability of presbycusis in families have been shown to be between 30-50%. However, presbycusis has been resistant to the traditional pattern of linkage analysis used to study families [5]. This is because ARHL has a high heterogeneity and as such, slight to moderate genetic variations and considerable environmental insult usually reduces the benefit of using a family genetics analysis approach [5]. Genetic research has been on the front burner in understanding Age related hearing loss (ARHL), which is the commonest sensory impairment in the elderly population [14]. The Human Genome Project which mapped all the genes that make up the human genome was a big leap in the history of human genetic research (Morton & Newman, 2019.). Following this project, genetic research has been employed to understand disease models, identify genes associated with human disease conditions, and to open new treatment targets for further investigation [14]. Due to the complexity of ARHL, there has been an increasing interest in genome wide association studies in humans, and in hearing loss associated genetic studies in mouse model. Due to the significant similarity between the human auditory system and that of mice or rat, and the relative inaccessibility of the auditory system in mammals, mice have been an extremely important model in the research for hearing loss in humans [2]. The advantage of controlling environmental exposures in mouse models, confers an improved replicability with a higher contribution to the hearing traits [7].
Erway et al in their research on inbred mice identified Age related hearing loss gene (AHL-1) on chromosome 10, which was associated with the degeneration of the organ of Corti, stria vascularis, spiral ligament and spiral ganglion. They further identified a second (AHL-2) and third (AHL-3) loci on chromosome 5 and chromosome 17 respectively [7]. Mitochondrial deletions known as common deletions (CD) have also been associated with hearing loss, in a way that temporal bone studies in humans suggest a correlation between the level of CD in the cochlea to the severity of hearing loss [15]. In human studies, several gene associated with presbycusis has been identified in several GWAS, like the study carried out in British population by Wells et al. [14] that identified 44 genes related to self-reported hearing loss and use of hearing aids. Over the last decade, several GWAS has tried to identify gene variants for ARHL, but none of these suggested significant loci have been successfully replicated [16]. This experience of the several GWAS carried out without a successful replication of identified loci buttresses the complexity of Presbycusis and highlights the variability of hearing loss genes. Hearing loss genes have various patterns of inheritance, which can be autosomal recessive, autosomal dominant, X-linked, or Mitochondrial form of inheritance. There is usually a correlation between the gene affected, and the peculiar phenotypic features seen clinically which can either be the age of onset, severity of hearing loss, frequencies affected, or the progression of the disease [17]. The hearing loss seen in affected individuals can be syndromic or non-syndromic. Syndromic hearing loss (SHL) is when hearing loss in an individual is associated with pathologies concomitantly present in other parts of the body, while Non-Syndromic hearing loss (NSHL) refers to hearing loss in the absence of any other associated pathology in the extra-auricular organs of the individual. NSHL is the common form of hearing loss and occurs in about 70% of congenital hearing loss cases [17]. Autosomal recessive inheritance has been noted to be responsible for majority of prelingual onset hearing loss, which usually affects speech and language development, whereas autosomal dominant inheritance has been implicated in post lingual hearing loss. A Non-syndromic autosomal dominant form of inheritance has been implied for ARHL, and there may be digenic or polygenic form of genetic expression that can promote this pathology in an individual, which can be modified by epigenetic factors like lifestyle and the environment [18]. This indicates the importance of precise identification of clinical phenotypes and a thorough family history in establishing a list of candidate genes in persons with hearing loss [19].
Genotype and Phenotypes have not always been consistent in predicting hearing disorders [19]. This phenotypic overlap due to the genetic and phenotypic heterogeneity, has led to the development of software that can utilize phenotypic and audiometric characteristics to determine the genetic constitution of an individual regarding their peculiar type of hearing loss. The development of hearing loss analytic software like the Audiogene and the APS programmes have encouraged genetic studies in hearing loss using a computer-based programme [20]. Since presbycusis is largely sensorineural and non-syndromic, Audiogene software has been tried in the study of age-related hearing loss, but there have been challenges associated with the use of these software in the genetic study of presbycusis. These challenges bother on the accuracy and reliability of the results obtained using genetic hearing loss analytic software like Audiogene [21]. As much as these software’s seem to be limited by the amount of data in their database, the greatest task may be in identifying subjects with genetic hearing loss like presbycusis from among a typical mixed cohort seen in the audiology clinic some of which have hearing loss due to acquired causes.
Presbycusis and Lifestyle Medicine
In the contemporary practice of medicine, as much as artificial intelligence has found a role in the diagnosis and treatment of pathological conditions, there is a concurrent inclination towards preventive medicine, with the propagation of lifestyle medicine through the promotion of a healthy lifestyle. This aspect of medicine promotes the use of dietary modification, good sleep, exercise, avoidance of harmful lifestyle practices like smoking, sedentary lifestyle, and reduction of physical or emotional stressors to boost immunity which invariably suppresses genetic predisposition to disease via epigenetic properties. Systemic illnesses like diabetes mellitus, Hypertension, and hyperlipidemia have been implicated in worsening hearing acuity in the elderly. Lifestyle medicine has been employed in the management of these medical conditions in the bid to control or reverse their complications, which includes hearing loss [22]. Spankovich and Le Prell in their study in 2014, examined the association of hearing threshold with assessment of a person’s compliance with the recommended dietary intake, termed the Healthy Eating Index (HEI). They reported that a healthier eating correlates to an improved hearing threshold especially at high frequencies [23]. Antioxidants have also been shown to improve hearing threshold especially in noise induced hearing loss and ototoxicity [24].
However, the effect of this has been difficult to ascertain in Presbycusis unlike other hearing loss with acute injury, because of its characteristic chronic gradual deterioration of hearing loss. Han et al studied the effect of exercise on hearing threshold in mice and reported that exercise improves hearing loss, evidenced by histologic and audiometric evaluation [25]. Cross-sectional and longitudinal studies in humans aimed at ascertaining the effect of lifestyle medicine principles on hearing thresholds have shown promising results. However, in presbycusis there are challenges because lots of confounding factors are encountered. This shows the complexity of ARHL, but most importantly, shows the difficulty in separating the extent to which each of genetics, environment and lifestyle contribute to the aetiology of presbycusis [26]. There is need to explore the benefits of a healthy or improved lifestyle practice on presbycusis through elaborate longitudinal research, to elucidate the role of epigenetics in delaying or curbing genetic hearing loss [27].
Conclusion
There is an overwhelming interest, with reasonable insight gained into the pathophysiology and genetics of ARHL, but the interaction of genetics, lifestyle, and environment certainly complicates our ability to separate their individual contributions to this pathology. There is need to improve research in the role of lifestyle medicine in curbing presbycusis.
References
- Lee KY (2013) Pathophysiology of age-related hearing loss (peripheral and central). Korean J Audiol 17(2): 45-49.
- Martines F, Maira E, Ferrara S (2011) Age-related Hearing Impairment (ARHI): A Common Sensory Deficit in the Elderly. Acta Medica Mediterranea 27: 47-52.
- Quaranta N, Coppola F, Casulli M, Barulli MR, Panza F, et al. (2015) Epidemiology of Age-related Hearing loss: A Review. Hearing, Balance and Communication Early Online p. 1-5.
- Dawes P, Payton A (2016) Genetics of age-related hearing loss in Genetics of Deafness Vona B, Haaf T (eds): monogr Hum Genet. Basel Karger 20: 84-96.
- Bowl MR, Dawson SJ (2019) Age Related Hearing Loss. Cold Spring Harb Perspect Med 9: a033217.
- Nagtegaal AP, Broer L, Zilhao NR, Jakobdottir J, Bishop CE, et al. (2019) Genome wide association meta-analysis identifies five novel loci for age-related hearing impairment. Nature Scientific report 9: 15192.
- Tu NC, Friedman RA (2018) Age-related Hearing loss: Unravel the Pieces. Laryngoscope investig Otolaryngol 3(2): 68-72.
- Jung DK, Kim D, Joo KM, Yang HS, Lee WB, et al. (2005) Age related changes in the distribution of Kv1.1 and Kv1.3 in rat cochlear nuclei. Neurol Res 27(4): 436-440.
- Idrizbegovic E, Canlon B, Bross LS, Willott JF, Bogdanovic N (2001) The total number of neurons and calcium binding protein positive neurons during aging in the cochlear nucleus of CBA/CAJ mice: a quantitative study. Hear Res 158:102–115.
- Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging Res 330: 191-199.
- Gates GA, Mills JH (2005) Presbycusis. The lancet 366(9491): 1111-1120.
- Huyghe JR, Laer LV, Hendrickx JJ, Fransen E, Demeester K, et al. (2008) Genome-wide SNP-based linkage scan identifies a locus on 8q24 for an age-related hearing impairment trait. The American Journal of Human Genetics 83(3): 401-407.
- Lewis MA, Nolan LS, Cadge BA, Matthews LJ, Schulte BA, et al. (2018) Whole exom sequencing in adult-onset hearing loss reveals a high load of predicted pathogenic variants in known deafness-associated genes and identifies new candidate genes. BMC Medical Genomics 11(77): 1-12.
- Wells RRH, Freudian BM, Abidin FNZ, Payton A, Dawes P, et al. (2019) GWAS identifies 44 independent loci for self-reported adult hearing difficulty in UK bio bank. American journal of human genetics 105: 788-802.
- Markaryan A, Nelson EG, Hinojosa R (2009) Quantification of the mitochondrial DNA common deletion in Presbycusis. Laryngoscope 119: 1184-1189.
- Hoffman TJ, Keats BJ, Yoshikawa N, Schaefer C, Risch N, et al. (2016) A Large Genome-Wide Association study of Age-related hearing impairment using electronic health records. PLOS Genetics 12(10): 1006371.
- Smith SD (2001) Relationships between neurologic disorders and hereditary hearing loss. Semin Pediatr Neurol 8(3): 147-159.
- Kremer H (2019) Hereditary hearing loss; about the known and the unknown. Hearing Research 376: 58-68.
- McDermott J, Molina-Ramírez L, Bruce L, Mahaveer A, Turner M, et al. (2019) Diagnosing and Preventing Hearing Loss in the Genomic Age. Trends in Hearing p. 23.
- Hildebrand M, Deluca A, Taylor K, Hoskinson D, Hur I, et al. (2019) A Contemporary Review of AudioGene Audioprofiling: A Machine-Based Candidate Gene Prediction Tool for Autosomal Dominant Nonsyndromic Hearing Loss. The Laryngoscope 119: 2211-2215.
- Weininger O, Warnecke A, Lesinski-Schiedat A, Lenarz T, Stolle S (2019) Computational analysis based on audioprofiles: A new possibility for patient stratification in office-based otology. Audiology Research 9: 230.
- Bainbridge KE, Hoffman HJ, Cowie CC (2011) Risk factors for hearing impaired among U.S. adults with diabetes. National Health and Nutrition Examination Survey 1999 – 2004. Diabetes Care 34: 1540-1545.
- Spankovich C, Le Prell CG (2014) Associations between dietary quality, noise, and hearing: data from the National Health and Nutrition Examination Survey, 1999–2002. Int J Audiol 53: 796–809.
- Seidman MD (2000) Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope 110: 727–738.
- Han C, Ding D, Lopez MC (2016) Effects of long-term exercise on agerelated hearing loss in mice. J Neurosci 36: 11308–11319.
- Erway LC, Willott JF, Archer JR, Harrison DE (1993) Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hearing Research 65 (1–2): 125-132.
- Lee KY (2013) Pathophysiology of age-related hearing loss (peripheral and central). Korean J Audiol 17(2): 45-49.
- Martines F, Maira E, Ferrara S (2011) Age-related Hearing Impairment (ARHI): A Common Sensory Deficit in the Elderly. Acta Medica Mediterranea 27: 47-52.
- Quaranta N, Coppola F, Casulli M, Barulli MR, Panza F, et al. (2015) Epidemiology of Age-related Hearing loss: A Review. Hearing, Balance and Communication Early Online p. 1-5.
- Dawes P, Payton A (2016) Genetics of age-related hearing loss in Genetics of Deafness Vona B, Haaf T (eds): monogr Hum Genet. Basel Karger 20: 84-96.
- Bowl MR, Dawson SJ (2019) Age Related Hearing Loss. Cold Spring Harb Perspect Med 9: a033217.
- Nagtegaal AP, Broer L, Zilhao NR, Jakobdottir J, Bishop CE, et al. (2019) Genome wide association meta-analysis identifies five novel loci for age-related hearing impairment. Nature Scientific report 9: 15192.
- Tu NC, Friedman RA (2018) Age-related Hearing loss: Unravel the Pieces. Laryngoscope investig Otolaryngol 3(2): 68-72.
- Jung DK, Kim D, Joo KM, Yang HS, Lee WB, et al. (2005) Age related changes in the distribution of Kv1.1 and Kv1.3 in rat cochlear nuclei. Neurol Res 27(4): 436-440.
- Idrizbegovic E, Canlon B, Bross LS, Willott JF, Bogdanovic N (2001) The total number of neurons and calcium binding protein positive neurons during aging in the cochlear nucleus of CBA/CAJ mice: a quantitative study. Hear Res 158:102–115.
- Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging Res 330: 191-199.
- Gates GA, Mills JH (2005) Presbycusis. The lancet 366(9491): 1111-1120.
- Huyghe JR, Laer LV, Hendrickx JJ, Fransen E, Demeester K, et al. (2008) Genome-wide SNP-based linkage scan identifies a locus on 8q24 for an age-related hearing impairment trait. The American Journal of Human Genetics 83(3): 401-407.
- Lewis MA, Nolan LS, Cadge BA, Matthews LJ, Schulte BA, et al. (2018) Whole exom sequencing in adult-onset hearing loss reveals a high load of predicted pathogenic variants in known deafness-associated genes and identifies new candidate genes. BMC Medical Genomics 11(77): 1-12.
- Wells RRH, Freudian BM, Abidin FNZ, Payton A, Dawes P, et al. (2019) GWAS identifies 44 independent loci for self-reported adult hearing difficulty in UK bio bank. American journal of human genetics 105: 788-802.
- Markaryan A, Nelson EG, Hinojosa R (2009) Quantification of the mitochondrial DNA common deletion in Presbycusis. Laryngoscope 119: 1184-1189.
- Hoffman TJ, Keats BJ, Yoshikawa N, Schaefer C, Risch N, et al. (2016) A Large Genome-Wide Association study of Age-related hearing impairment using electronic health records. PLOS Genetics 12(10): 1006371.
- Smith SD (2001) Relationships between neurologic disorders and hereditary hearing loss. Semin Pediatr Neurol 8(3): 147-159.
- Kremer H (2019) Hereditary hearing loss; about the known and the unknown. Hearing Research 376: 58-68.
- McDermott J, Molina-Ramírez L, Bruce L, Mahaveer A, Turner M, et al. (2019) Diagnosing and Preventing Hearing Loss in the Genomic Age. Trends in Hearing p. 23.
- Hildebrand M, Deluca A, Taylor K, Hoskinson D, Hur I, et al. (2019) A Contemporary Review of AudioGene Audioprofiling: A Machine-Based Candidate Gene Prediction Tool for Autosomal Dominant Nonsyndromic Hearing Loss. The Laryngoscope 119: 2211-2215.
- Weininger O, Warnecke A, Lesinski-Schiedat A, Lenarz T, Stolle S (2019) Computational analysis based on audioprofiles: A new possibility for patient stratification in office-based otology. Audiology Research 9: 230.
- Bainbridge KE, Hoffman HJ, Cowie CC (2011) Risk factors for hearing impaired among U.S. adults with diabetes. National Health and Nutrition Examination Survey 1999 – 2004. Diabetes Care 34: 1540-1545.
- Spankovich C, Le Prell CG (2014) Associations between dietary quality, noise, and hearing: data from the National Health and Nutrition Examination Survey, 1999–2002. Int J Audiol 53: 796–809.
- Seidman MD (2000) Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope 110: 727–738.
- Han C, Ding D, Lopez MC (2016) Effects of long-term exercise on agerelated hearing loss in mice. J Neurosci 36: 11308–11319.
- Erway LC, Willott JF, Archer JR, Harrison DE (1993) Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hearing Research 65 (1–2): 125-132.
- Taylor K, Deluca A, Shearer A, Hildebrand M, Black-Ziegelbein E, et al. (2013) AudioGene: Predicting Hearing Loss Genotypes from Phenotypes to Guide Genetic Screening. Human mutation 34(4): 539-545.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...