
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Case Report(ISSN: 2641-1709) 
Nasal Septal Schwannoma: a Case Report and Review of the Literature Volume 10 - Issue 2
Florent Jamme1*, Anaïs de Bont2, Françoise Hulet3 and Philippe Eloy4
- 1Department of ENT and Head & Neck surgery, Cliniques Universitaires Saint Luc, Belgium
- 2Department of ENT and Head & Neck surgery, University Hospital Ambroise Paré, Belgium
- 3Pathology Laboratory, University Hospital Ambroise Paré, Belgium
- 4Department of ENT and Head & Neck surgery, CHU UCL Namur site de Godinne, Belgium
Received:June 28, 2023; Published:July 07, 2023
Corresponding author:Florent Jamme, Department of ENT and Head & Neck surgery, Cliniques Universitaires Saint Luc, Avenue Hippocrate, B-1200 Woluwe Saint-Lambert, Brussels, Belgium
DOI: 10.32474/SJO.2023.10.000332
The authors report the story of a schwannoma implanted on the left side of the nasal septum in a 46- year-old female patient complaining of nasal obstruction. A sinus CT-scanner showed a non- encapsulated 32 x 28 x 18 mm soft-tissue mass in the left nasal fossa, associated with a left anterior sinusitis. Biopsy didn’t show any sign of malignancy. The tumour was resected endoscopically associated to a middle antrostomy and an ethmoidectomy under general anaesthesia. Pathological and immunohistochemical analysis gave the final diagnosis. With a follow up of 4 years, there was no recurrence. Interestingly, the patient reported the resection of another schwannoma originated from the median hard palate 28 years earlier. The authors report the case and review the worldwide literature on this rare lesion.
Introduction
Schwannomas are rare, mostly benign neoplasms constituted of proliferative differentiated Schwann cells from myelinated nerve sheaths, derived from the neural crests. They usually present as well-circumscribed and frequently encapsulated slow-growing solitary tumors. 90% of schwannomas are sporadic. They can on occasion be multiple or recurrent, either in cases of incomplete resection, or in certain genetic conditions.
They originate from any myelinated nerve (somatic, autonomic and cranial nerves) except for olfactory and optic nerves who don’t have any Schwann cell, but very similar olfactory unsheathing cells derived from the olfactory placode. On the nasal septum, this concerns autonomic fibres heading to the submucosal glands and vessels or trigeminal maxillary and ophthalmic branches mediating somatosensory signals from the mucosa through the naso- palatine, great palatine and naso-ciliary nerves. Schwannomas constitute approximately 5% of all tumors of the head and neck, which hosts 25 to 45% of them. Less than 4% of schwannomas are found in the sinonasal area1, while up to 80% arise from the vestibular nerve. In the nose and paranasal sinuses, the most frequent sites are the ethmoidal sinus, the maxillary sinus, and finally the sphenoid sinus. Schwannomas originating from the nasal septum are exceedingly rare.
Case Report
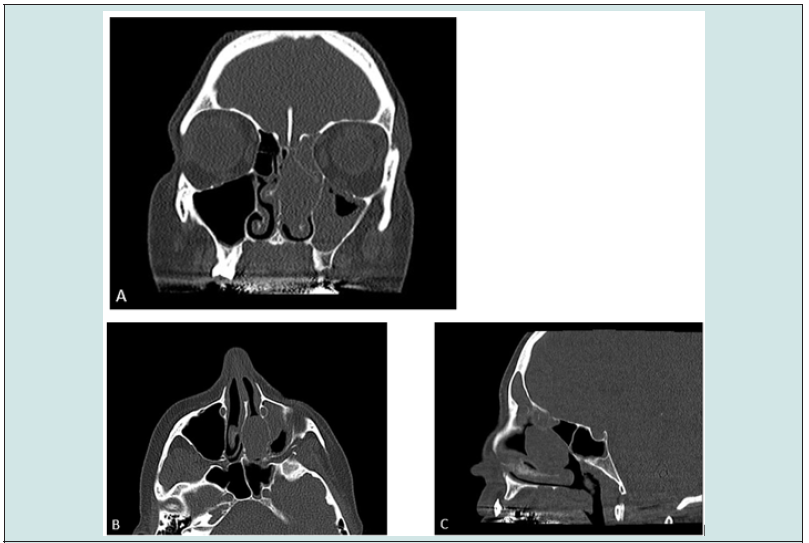
A 46-year old Mediterranean woman first consulted in pneumology for sleeping disturbance. She had unilateral nasal obstruction with an important rhinolalia and left muco-purulent rhinorrhea. There was no complaints of epistaxis, facial pain, facial swelling or hyposmia. In her medical history, we noticed an allergic asthma and allergic rhinitis, no chronic sinusitis, nasal surgery or smoking history. She reported the resection of a schwannoma from the median hard palate 28 years before. A non-contrast-enhanced computed tomography (CT) of the paranasal sinuses revealed a 32 x 28 x 18 mm homogenous non- encapsulated soft tissue density in the left nasal cavity and olfactory fossa (Figure 1). The nasal septum deviated on the right side. The left middle turbinate was lateralized and there was an associated left anterior pansinusitis. There was no bone erosion. The patient was referred to the Otorhinolaryngology outpatient clinic. On examination, a well-circumscribed submucosal mass with light and smooth overlying mucosa was observed occupying the left nasal cavity. A biopsy was performed endoscopically under local anaesthesia. Microscopical analysis of the sample didn’t show any malignant characteristic, despite not being formally identified.
Figure 1: Non contrast-enhanced CT scanner of the paranasal sinuses in bony window. A - coronal image : well- demarcated tissular mass in the left nasal fossa, osteomeatal complex obstruction, no orbital or cranial damage. B - axial image : no bone erosion, free lachrymal duct. C - sagittal image : posterior situation of the lesion. Left anterior sinusitis.
The mass was then removed endoscopically under general anesthesia with resection of the septal mucoperiochondrium. The resection was complete with safe surgical margins. The site of insertion was the posterior portion of the nasal septum. The authors combined the resection to a left middle antrostomy and left ethmoidectomy. The histological analysis of the specimen (Figure 2) showed bundles of spindle-shaped cells with fusiform nuclei showing focal atypia, arranged into a dense organized cellular cytoplasm with a discrete palisading pattern. There were also alternating areas of normal respiratory and sinusal epithelia with a slightly inflammatory lympho-plasmocytic infiltrate in a looser hypocellular chorion. No evidence of necrosis was found into the analyzed specimen. Immunohistochemical staining was diffusely and strongly positive for S100 protein and negative for desmin, actin and epithelial membrane antigen (EMA). The final diagnosis was a benign schwannoma of the nasal septum. Post-operative evolution was uneventful. A postoperative MRI performed 6 months after surgery confirmed the absence of residual tumour. 4 years after the intervention, the patient is still free of disease. The authors wanted to refer the patient to a geneticist because this was the second resection of a schwannoma in this patient, but she refused.
Figure 2: Anatomopathological analysis of the specimen. A - Antoni A palisading pattern forming Verocay bodies dispersed in a loose chorion (Antoni B). B - Immunohistochemical staining shows diffuse positivity for S-100 protein. C - clear delineation between the nasal respiratory epithelium and the submucosal mass.

Literature Review
The authors reviewed different databases (PubMed, Research gate, Europe PMC) with the terms: “nasal septal schwannoma”, “nasal septum schwannoma” and “nasal septal neurinoma”. They found 35 reported cases between January 2000 and September 2020 in the available English scientific literature. Sino- nasal schwannomas are more commonly reported. In this review, there was no gender preponderance. The tumour arises in adults with ages ranging from 16 to 82 years old. The diagnosis is generally suspected by nasal endoscopy and the extension is defined by imaging: typically a contrast- enhanced CT scan and sometimes an MRI. Bone erosion is often seen, despite the benign character of these tumours, leading to an erroneous suspicion of malignancy [1] (Table 1).
Table 1: Literature review : all nasal septal schwannomas reported in the available English literature between 2000 and 2020.

M = male ; F = female ; L = left ; R = right ; CT = computed tomography ; MRI = magnetic resonance imaging ; NED = no evidence of disease.
Discussion
Although schwannomas are benign tumours, they have a significant comorbidity, mostly due to a mass effect leading to a bone erosion onto neighboring structures. The symptoms depend on the anatomical location of the lesion and on its size. In the nasal cavity, the most frequent symptoms are unilateral nasal obstruction, epistaxis, facial pain and sometimes nasal discharge. In long-evolving cases, more debilitating symptoms can occur, such as exophthalmos, diplopia, facial swelling, cranial nerve deficit, skull base fistula, hyposmia, epiphora or hypopituitarism [2]. In the nasal cavity and nasal septum, they tend to become symptomatic more quickly than in the paranasal sinuses. However, when it originates from the posterior part of the nasal septum the extension usually progresses towards the sphenoethmoidal recess, or the nasopharynx with a long asymptomatic period, which can cause a delay in diagnosis. Because the symptomatology and the macroscopic and endoscopic aspects of most intranasal masses are not specific, imaging and histology are mandatory to make the definitive diagnosis.
Imaging
Imaging techniques for sinonasal tumours include mainly
cross-sectional CT (to evaluate thickening, remodeling, erosion,
sclerosis or destruction of adjacent bone) and MRI (to characterize
the tissular components of the mass, its extent, to rule out the
presence of pathologic lymph nodes and to differentiate the lesion
itself from retention sinusitis)[3]. Intravenous contrast medium
injection for both imaging techniques is recommended in nasal tumours
evaluation protocols. Schwannomas appear in tomography
as well- delineated, often capsulated soft-tissue tumours with a
local extension that can induce mucous retention and sometimes
bone deformation or bone destruction by mass effect [1]. In gadolinium-
enhanced multiplanar MRI, schwannomas are hypointense
or isointense to skeletal muscles in T1-weighted images, and heterogeneously
hyperintense in T2-weighted acquisitions, due to the
alternance of Antoni A and Antoni B areas [3]. This pattern is not
specific of nerve sheath tumours, but this category shares other
specific features :
a. The “target sign”, characterized by low- signal intensity
with low contrast enhancement centrally and high-signal
intensity with high contrast enhancement peripherally on
T2-weighted and contrast- enhanced T1-weighted images, has
a high specificity but a low sensitivity [4].
b. The “fascicular sign” shows a fascicular bundles pattern as
in normal large diameter nerves.
c. If the affected nerve trunk is large, it can sometimes be
seen at the periphery of the mass for schwannomas, but by far
less frequently in neurofibromas, in which the affected nerve
passes through the tumour and is often indistinguishable [5].
In the nose and paranasal sinuses, the nerve of origin is seldom
identified due to their relatively small diameter.
In the present case, MRI wasn’t performed preoperatively because it wouldn’t have added any important information after CT. The latter was sufficient to rule out involvement of any vital structure and it gave clues towards a benign mass, easily removable in toto.
PET-CT can be used complimentarily in selected cases suspicious for malignancy, for N- and M- staging, to monitor the response to a treatment or to screen for residual or recurrent tumours. It is, however, not recommended for routine diagnosis [3].
Histology
Histopathological examination, often helped by immuno-histochemistry (IHC), is the only way to obtain a definitive diagnosis for tumours of the nose and paranasal sinuses. A biopsy of the mass is recommended before surgery. For significant tumours the biopsy must be performed under general anaesthesia. Typically these tumours have a trend to bleed. Schwannomas stained by haematoxylin and eosin show under optic microscopy a binary aspect of alternating Antoni A and Antoni B areas in various combinations. The former is made of compact indistinguishable spindle cells with twisted nuclei arranged in bundles of interlacing fascicles. This nuclear palisading pattern around a central acellular hypereosinophylic area defines a “Verocay body” [6]. Antoni B areas are by far less cellular with a loose myxoid stroma and occasionally glands or benign epithelial structures [2]. In addition, there is thickening and hyalinization of blood vessels’ walls.
Immunohistochemistry
IHC of schwannomas shows a diffuse and intense positivity of the cytoplasm and nuclei for S-100 protein : a neural-crest antigen of the supporting cells of the nervous system which is specific to Schwann cells and melanocytes [7]. Thus, S-100 positive staining also applies to melanoma, meningioma or neurofibroma. Vimentin, an ubiquitarian protein of mesenchymal origin, is negative for schwannomas but not for other mesenchymal (sarcomas) and spindle- cell neoplasms. Smooth muscle actin (SMA) negativity helps to rule out leiomyoma. Desmin is a mesenchymal marker of myogenic origin to which smooth muscle tumours are inconstantly positive EMA (Epithelial membrane antigen) and PgR (progesterone receptor) are both good markers for meningiomas [8]. The S-100 marker usually stains less diffusely and less intensely into neurofibromas than in schwannomas. Calretinin is expressed in 96% of schwannomas versus only 7% of neurofibromas. In peripheral nerves, CD34 is expressed by dendritic and endoneural fibroblastic cells. In schwannomas, CD34 staining underlines the biphasic pattern by being mostly present in Antoni B areas. On the contrary, CD34 is more diffuse in neurofibromas with no biphasic pattern [9].
Differential diagnosis
Although they are not exhaustive, these case series published by Bist [10] and Yildirim [11] give a broad overview of the different tumours often encountered in the nose. Histological assessment is therefore of utmost importance to make a differential diagnosis. The most important lesions to rule out are neurofibromas and malignant transformation. Neurofibromas are more prone to malignant evolution (incidence of 2,4% to 16,5%) than schwannomas and are more locally aggressive. Complete resection of schwannomas is easier as they usually have a true capsule, and their originating nerve stays at the periphery. On the contrary, neurofibromas are less prone to have a capsule and frequently englobe the nerve [12]. Malignant schwannomas can occur either as an evolution of a previously benign schwannoma, of a neurofibroma or de novo. Malignant transformation is extremely rare except in Von Recklinghausen’s disease [13], where their incidence raises up to 10-15%. Until now, no case of malignant schwannoma from the nasal septum has been reported. Microscopically, signs of malignancy are focal hyperchromasia and dense packing with increased cellularity, indistinct cytoplasm, slender wavy or curled nuclei with numerous mitoses, anaplastic cells and invasiveness. IHC of Malignant schwannomas shows positive staining for S-100 antigen, Leu-17, vimentin and CD56 and negative staining for CK, LCA, CD34, SMA, desmin, chromogranin, and synaptophysin [14].
Genetics
Some genomic mutations associated with schwannomas are responsible for a group of genetic neurocutaneous disorders characterized by a predisposition to nerve sheath tumours called neurofibromatosis (type 1, type 2 and schwannomatosis) and Carney complex. Despite sharing some phenotypical similarities, their genotypical, molecular and clinical features distinguish those diseases. Nevertheless, schwannomas are often solitary and sporadic. An integrative genetic analysis of 125 vestibular and spinal schwannomas assessed their genomic basis [15]. It put in light that NF2 gene was altered or deleted in 76% of cases. In our patient, NF1 and NF2 didn’t seem very likely. Indeed, given the absence of family history, her age and the full penetrance of these clinically diagnosed conditions, it seems reasonable to consider that these affections would already have been diagnosed. It was, however, impossible to tell if both schwannomas were the expression of a schwannomatosis trait, two unrelated sporadic events or a recurrence of the same incompletely resected lesion on her naso- palatine nerve, 28 years later. Nevertheless, this seems quite long for a recurrence, even for these slow-growing tumours.
Treatment
The gold standard treatment for sinonasal benign schwannoma is surgery: a complete resection with safe margins. Nowadays, the endoscopic endonasal approach is the modality of choice in the majority of cases. In fact the lesions are frequently of moderate size and well circumscribed. In very extensive lesions with extra-nasal extension, an external approach can be considered. The dissection should include the muco-perichondrium or the mucoperiosteum [16]. In case of very extensive lesions, inoperable patient or malignant transformation radiotherapy can be considered even if schwannomas are typically radioresistant.
Follow-up
There are no definitive guidelines concerning the follow-up of these lesions in the literature due to their rarity. However, it is reasonable to follow up the patient by nasal endoscopy during the 3 first years following the surgery. An additional imaging (CT or MRI) can be necessary in extensive lesions or when nasal endoscopy is not conclusive. In the literature, no recurrence has been reported in case of complete resection with safe margins. No malignancy transformation was reported either.
Conclusion
Schwannomas are peripheral nerve sheath tumours seldom encountered in the sinonasal area and exceptionally on the nasal septum. A proper clinical and radiological assessment is of utmost importance to make a preoperative mapping of the tumour. CT and MRI are the best imaging modalities. As these tumours are well circumscribed, endoscopic endonasal surgery is nowadays the gold standard treatment in the majority of cases. Anatomopathology with IHC is essential to make a precise and definitive diagnosis. Neurofibromas and sarcomas are the most common differential diagnosis.
References
- Dublin AB, Dedo HH, Bridger WH (1995) Intranasal schwannoma: magnetic resonance and computed tomography appearance. American journal of otolaryngology 16(4): 251-254.
- Donnelly MJ, Al Sader MH, Blayney AW (1992) Benign nasal schwannoma. The Journal of Laryngology & Otology 106(11): 1011-1015.
- Eggesbø HB (2012) Imaging of sinonasal tumours. Cancer Imaging 12: 136-152.
- Skolnik AD, Loevner LA, Sampathu DM, Newman JG, Lee JY, et al. (2016) Cranial nerve schwannomas: Diagnostic imaging approach [Article]. Radiographics 36(5): 1463-1477.
- Lemmerling M, Moerman M, Govaere F, Praet M, Kunnen M, et al. (1998) Schwannoma of the tip of the nose: MRI. Neuroradiology 40(4): 264-266.
- Arcot R, Ramakrishnan K, Rao S (2012) Peripheral and cranial nerve sheath tumors—a clinical spectrum. Indian Journal of Surgery 74: 371-375.
- Stefansson K, Wollmann R, Jerkovic M (1982) S-100 protein in soft-tissue tumors derived from Schwann cells and melanocytes. The American Journal of Pathology 106(2): 261.
- Boulagnon Rombi C, Fleury C, Fichel C, Lefour S, Marchal Bressenot A, et al. (2017) Immunohistochemical approach to the differential diagnosis of meningiomas and their mimics. Journal of Neuropatholgy & Experimental Neurology 76(4): 289-298.
- Ohashi R, Wakayama N, Kawamoto M, Tsuchiya S, Okubo K (2013) Solitary nasal schwannoma: usefulness of CD34 and calretinin staining for distinction from histological mimics. Journal of Nippon Medical School 80(4): 300-306.
- Bist SS, Varshney S, Baunthiyal V, Bhagat S, Kusum A (2012) Clinico-pathological profile of sinonasal masses: An experience in tertiary care hospital of Uttarakhand. National journal of maxillofacial surgery 3(2): 180.
- Yildirim D, Saglam O, Gurpinar B, Ilica T (2012) Nasal cavity masses: clinico-radiologic collaborations, differential diagnosis by special clues. Open Journal of Medical Imaging 2(1): 10.
- Valencia MP, Castillo M (2008) Congenital and acquired lesions of the nasal septum: a practical guide for differential diagnosis. Radiographics 28(1): 205-223.
- Perzin KH, Panyu H, Wechter S (1982) Nonepithelial tumors of the nasal cavity, paranasal sinuses and nasopharynx. A clinicopathologic study. XII: Schwann cell tumors (neurilemoma, neurofibroma, malignant schwannoma). Cancer 50(10): 2193-2202.
- Maheshwari G, Baboo H, Gopal U, Baiar D, Shah N (1999) Malignant schwannoma of the sinonasal tract. Indian Journal of Otolaryngology and Head & Neck Surgery 51: 47-50.
- Agnihotri S, Jalali S, Wilson MR, Danesh A, Li M, et al. (2016) The genomic landscape of schwannoma. Nature genetics 48(11): 1339-1348.
- Forer B, Lin LJ, Sethi DS, Landsberg R (2015) Endoscopic resection of sinonasal tract schwannoma: presentation, treatment, and outcome in 10 cases. Annals of Otology, Rhinology & Laryngology 124(8): 603-608.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




