Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6003
Research Article(ISSN: 2638-6003) 
Continuous Spinal Anesthesia with Spinocath® Catheter. A Retrospective Analysis of 455 Orthopedic Elderly Patients in the past 17 years Volume 4 - Issue 1
Luiz Eduardo Imbelloni1*, Marildo A. Gouveia2, Geraldo Borges de Morais Filho3, Jaime Weslei Sakamoto1, Eduardo Piccinini Viana1 and André Augusto de Araujo1
- 1Anesthesiologist of Hospital Clínicas Municipal de São Bernardo do Campo, Brazil
- 2Ex-President Latin America Society Regional Anesthesia, Brazil
- 3Master in Labour Economics, Brazil
Received: May 11, 2020; Published: May 20, 2020
Corresponding author: Luiz Eduardo Imbelloni, Anesthesiologist of Hospital Clínicas Municipal de São Bernardo do Campo, São Paulo, SP - Brazil.
DOI: 10.32474/OSMOAJ.2020.04.000178
Abstract
Background and Objectives: Database analysis in general cost less and require less time as compared to large randomized controlled trials. This retrospective study with a catheter outside the cutting-tip needle for continuous spinal anesthesia for femur and hip surgery in elderly patients from 1998 to 2015, with the aim of determine possible advantages and disadvantages of this technique.
Methods: Anesthetic records of 455 patients receiving continuous spinal anesthesia over a 17-year period were analyzed retrospectively. All blockades were performed with patients in the left lateral position and by the two authors. Doses of 0.5% isobaric bupivacaine were administered according to the patient’s height. Evaluated parameters were: puncture success, highest level of anesthesia, lower limb motor block, quality of anesthesia, need for additional doses, failures incidence, paresthesia, postdural puncture headache, cardiovascular changes, mental confusion and delirium, blood transfusion and mortality.
Results: Seven patients were excluded for failure to puncture and accidental perforation of the duramater. The mean time for puncture and placement of the catheter was 2.66±1.03 min. The kit was easy to use in 376 patients and difficult in 42 patients. In all patients the catheter was inserted from 1 to 2 cm in the subarachnoid space. The mode of dispersion cephalad analgesia was T12. In 360 patients, the initial dose was sufficient to reach T12 and 88 patients required to supplement the dose. Mean isobaric bupivacaine initial dose was 7.74±1.78 mg and total dose was 8.58±2.60 mg. Hypotension occurred in 32 patients and bradycardia in 21 patients. Low intensity headache lasting for 3 days has been observed in seven patients. There has been no cauda equina syndrome or transient radicular irritation. Mental confusion occurred in 29 patients.
Conclusions: Our results with 455 patients over 17 years suggest that continuous spinal anesthesia with the catheter outside the needle for elderly orthopedic patient’s shows minor insertion problem, a low incidence of hypotension, paresthesia and headache. No neurological complications were observed, such as cauda equina syndrome or transient neurological symptoms.
Keywords: Anesthetics; Local: Isobaric Bupivacaine; Anesthetic Techniques; Regional: Continuous Spinal Block; Surgery; Orthopedic
Introduction
With the appearance of microcatheters (calibers 28 to 32G)
in 1990 there was a resurgence of interest in continuous spinal
anesthesia (CSA) [1]. Microcatheters are difficult to handle,
the appearance CSF is slow or impossible, injection of the local
anesthetic is slow, can break and provide inadequate blocks due to
poor anesthetic distribution hyperbaric in the subarachnoid space,
which can cause cauda equina syndrome [2, 3]. In 1995, a new
spinal anesthesia catheter was used in Europe [4]. This 22G and 24G caliber catheter, 73 cm long, is mounted outside a spinal anesthesia
needle caliber 27G and 29G, with Quincke point. It has terminal
opening and only one side hole 0.5 cm from the tip, requiring only
an inch of your length is introduced into the subarachnoid space.
Three years after its initial use in Germany, it arrived in Brazil
and one year after, the first article was published with this new
catheter for CSA in 40 patients with orthopedic lower limb surgery,
suggesting CSA with the catheter outside the needle shows minor
insertion problems and a low incidence of hypotension [5].
Subsequently, we compared CSA with combined spinal-epidural
anesthesia and sing shot spinal anesthesia (SSA) in a retrospective
study [6] and compared with combined spinal-epidural anesthesia
in a prospective study [7], provided good surgical conditions with
a low mortality rate in the first postoperative month and to a low
incidence of complications. And finally in 2006, we used it for labor
analgesia with the 29G needle and 24G catheter set in five pregnant
patients [8]. The catheter to perform CSA arrived in Brazil in 1998
and was discontinued in 2016 by the company that marketed it.
In Brazil our group published several articles with the kit for CSA.
Thus, we retrospectively assessed the number of CSA performed by
our study group. Our objectives were to evaluate the use of CSA, its
efficacy, ease to use and safety over the 17 year period.
Method
After obtaining institutional approval and informed consent from the subjects, this retrospective analysis was conducted the period from June 1998 to December 2015. All patients who submitted to femur osteosyntesis and partial or total hip replacement and received CSA carried out in this period were noted in an Excel spreadsheet designed for this monitoring and were reviewed. Patients’ demographic profiles, ASA physical status, comorbidities and clinical outcome were noted in the Excel spreadsheet. Details of the CSA, performance parameters, duration of surgery, intraoperative hemodynamic status and the usage of vasopressor and atropine were obtained from the anesthesia records. Inclusion criteria are shown in (Table 1). Associated diseases and drugs in use were also recorded. No patient was premedicated. Monitoring in the operating room consisted of continuous ECG in CM5, non-invasive blood pressure and pulse oximetry. All patients had an upper limb vein punctured with an 18G venous catheter and a 3 L.min-1 oxygen catheter or Hudson mask installed. After venous puncture, patients were given intravenous midazolam (0.5-1 mg). To place the patient in the blockade position, 0.1 mg/kg dextroketamine IV were injected, or anterior plexus lumbar blockade was performed with 20 mL of 2% lidocaine with epinephrine 1:200.000 + 20 mL of 0.5% bupivacaine. In patients operated for partial or total hip arthroplasty, they received dextroketamine and posterior lumbar plexus block with 40 mL of 0.25% bupivacaine for postoperative analgesia. Using the previously described technique [5], the epidural puncture was paramedially performed in the left lateral position at L2-L3 or L3-L4 interspace with an 18G Crawford needle. After that, dura was punctured with a Spinocath® device (B. Braun Melsungen AG) with a 27G needle and 22G catheter set. With the patient still in the puncture position, 5 to 10mg of 0.5% isobaric bupivacaine was injected, depending on patient’s height, when they were immediately placed in the supine position (Table 2). The following data were recorded: time taken for catheter insertion, perception of dural puncturing by spinal needle, difficulty of technique (“easy”, “difficult”, “impossible” or “perforation duramater”), highest level of sensory blockade, quality of motor blockade according to the Bromage scale, incidence of paresthesia, duration of the surgical procedure and neurologic complications. In case of pain or inadequate level, 2.5 mg of 0.5% bupivacaine were injected through the spinal catheter, until problem correction, which was removed at the end of surgery.
If accidental dural puncture were to occur during attempts to use an epidural approach with Crawford or Tuohy needles, the catheter would have to be introduced into the subarachnoid space and such patients would be excluded from the study. In the event of failure to access the epidural space within 15 minutes, singleshot spinal anesthesia would be administered with 15 mg of 0.5% isobaric bupivacaine and such patients would be excluded. All anesthesia’s were performed by or in the presence of the two authors (LEI, MAG). Hypotension (defined as a 30% decrease in systolic blood pressure, in comparison with preoperative control levels) was treated with ethylphenylephrine 1 mg intravenously. Bradycardia (defined as HR less than 50beats/min) was treated with atropine 0.5 mg intravenously. The patients were followed up by telephone regarding the appearance of cauda equina syndrome or transient neurological symptoms. The results were evaluated by the descriptive analysis of studied variables (frequencies, percentages, scatter plots and concentration ellipses) and, when possible, by the mean and standard deviation
Results
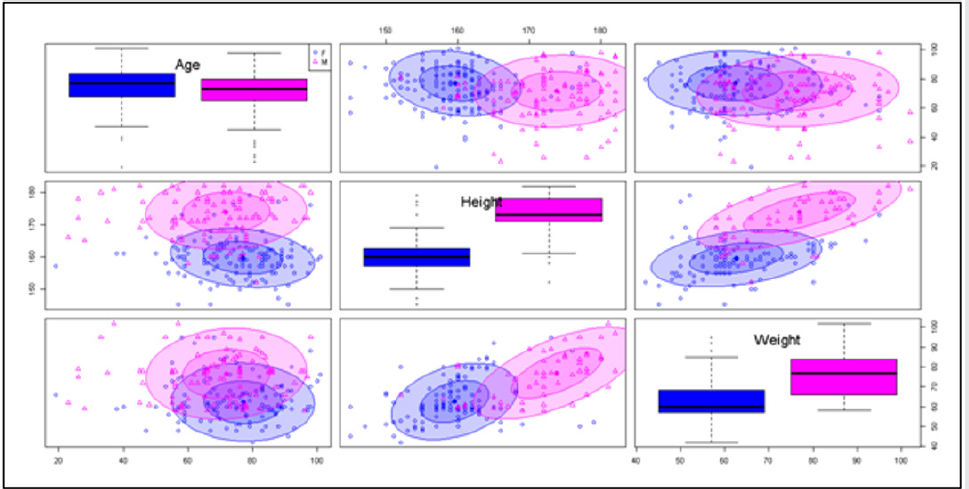
Four hundred and fifty-five underwent patient’s surgery using CSA during 17 years of the studied period. Of these, 298 (65.9%) were females. All of these CSA were carried out by the two authors. The 27G needle and 22G catheter were used in all patients. Only seven patients had to be excluded because of unintended dural perforation with the epidural needle in two patients or failure to access the epidural space with the Crawford needle in five patients. Demographic data are shown in (Table 3) and (Figure 1). The different doses used in the 448 patients are shown in (Table 4). Mean isobaric bupivacaine initial dose was 7.74±1.78 mg and total dose was 8.58±2.60 mg. The time to perform CSA was 2.36±1.03 minutes and the duration of surgery was 2.17±0.82 hours. In 376 patients, epidural puncture with Crawford needle was easy; in 72 patients it was difficult. The subarachnoid catheter was inserted easily in 407 patients and with difficulty in 42 and in all patients the catheter was inserted only 1 to 2 cm in the subarachnoid space. Paresthesia was observed in only 27 patients. In the seven patients where there was accidental perforation of the dura mater or failure to identify the epidural space, simple spinal anesthesia was performed with 15 mg of 0.5% isobaric bupivacaine (Table 5).
The cephalic dispersion of anesthesia was observed between T12 and T5 and the mode obtained was in T12 (Table 5). In 360 patients, the dose programmed according to height was sufficient to perform the procedure. There was a need for a supplementary dose in 88 patients due to the level and/or insufficient time to perform the surgery. Maximum motor block (Bromage 3) was observed in 340 patients. The initial degrees of motor block are shown in (Table 5). Arterial hypotension was observed in 32 patients (7.1%) and bradycardia in 21 patients (4.6%) who required treatment with vasopressors and atropine. Low-intensity headache and lasting three days was observed after CSA in seven patients (1.5%). Twenty-nine patients presented postoperative mental confusion. All catheters were removed at the end of the surgery and there was no presence of CSF in the dressing. There was no case of cauda equina syndrome or transient neurological symptoms. Most patients had multiple comorbidities. The most common comorbidities are hypertension (48%), diabetes mellitus (22%), moderate kidney disease (7%), chronic pulmonary disease (9%) and congestive cardiac failure (5%). Fourteen Jehovah’s Witnesses’ patients participated in the study, no need for blood transfusion.
Discussion
This retrospective study has shown that for femur and hip
surgeries in elderly patients, CSA with catheter designed for
this procedure provides less cephalad dispersion (mode T12),
lower incidence of arterial hypotension and less local anesthetic
requirement, without any neurological complications. The failure
rate was low (1.5%) and need for complementation of the initial
dose of 19.6%. Femur and hip fractures are major issues for health
services. Incidence increases with age, with predominance of
women due to association to osteoporosis. In our study, this was
confirmed by the 65.9% presence of women in the groups. The
utilization rate of CSA technique only in elderly patients with hip
or hip fracture by our group for 17 years averaged 30 patients per
year [5-7]. In 2006, we used the set (24G catheter and 29G needle)
for labor analgesia in five parturients, with excellent results [8]. In
2016 we stopped using CSA with Spinocath® has been discontinued
from the market.
In a previous study comparing CSA with continuous epidural
anesthesia (CEA), the time to perform it was significantly shorter
with CSA (2.6±0.9 min) than with CEA (2.9±1.2 min) [7]. In this
retrospective study with 448 patients, the time to perform the CSA
was shorter (2.36±1.03 min) than that obtained in the previous
article. Using the same kit for CSA (n=50) compared to CEA, the
performance time was significantly longer with CSA (6.09±2.20
min) and practically three times that obtained in our studies [9].
In most of our patients, they received an inguinal lumbar plexus
block before CSA. For this reason, we do not evaluate the latency
time of the first dose of 0.5% isobaric bupivacaine. In a study
comparing CSA versus CEA, the time to reach sensory level T10
was significantly lower with CSA (8.40±3.96 min x 18.80±6.59 min)
[9]. Sensory block level and motor blockade may be easily obtained
and controlled with CSA, in the same way allows early recognition
of insufficient level or insufficient time for the surgical procedure.
Because of the incremental doses in 19.6% of the patients, either
to produce the required analgesia or to extend analgesia, it would
be useless to study the final dermatome level of analgesia. CSA was
introduced in 1907 [10]. It is a well-established technique that has
been used successfully in orthopedic surgical procedures [5-7]. The
technique allows titration of the local anesthetic dose according to
surgical needs and provides safe anesthesia, particularly for elderly
or high-risk patients with unstable hemodynamic status [5, 11]. CSA
depends on how the catheter is introduced into the subarachnoid
space. It is more difficult when a microcatheter is used [1-3]. We
found difficulties during catheter insertion in 9.2% of the patients
in the CSA group, an incidence 3.6 times higher than in a previous
study [7]. In three studies comparing CSA with CEP, it showed a
significantly lower dose in the CSA group [6, 7, 9]. The total mean
dose of 0.5% isobaric bupivacaine was similar in the three studies
and practically the same in this group of 448 patients (8.58±2.60
mg).
In a recent study, it was found that CSA took longer with a
Spinocath® with 29G Quincke needle and 24G catheter (6.3±3.2
min) than with a microcatheter 22G Sprotte needle and 27G
catheter (3.9±1.2 min) [12]. This time was 2.6 times longer than
what we found in our study, using the Spinocath® with 27G Quincke
needle and 22G catheters. It is well known that the time taken for
cerebrospinal fluid to flow through a 29G needle with Quincke
bevel (80.45 seconds) is three times longer than through a 27G
needle (27.21 seconds) [13]. The use of different types and sizes of
needles may explain this difference. Some studies used the CSA for
post-operative analgesia for abdominal, vascular, hip surgery [14]
and severe aortic stenosis with hip fracture [15]. Because we used
lumbar plexus block (anterior and posterior) with neurostimulator,
the use of CSA for postoperative analgesia was not practiced in our
routine and all catheters were removed at the end of surgery. CSA
using small titrated dose provides better hemodynamic stability
than SSA [6] and CEA [6, 7] in elderly orthopedic patients. Although
transoperative hypotension (7.1%) may occur, it was easily treated
with small doses of vasopressors without any major adverse event
reported. Because it has a larger diameter than the needle 27G and
the catheter 22G occludes the duramater orifice and prevents CSF
loss and develops a reaction with fibrin deposit at the puncture site, which has already been shown to be animal [11]. There was
no presence of CSF in the dressing during the removal of the
catheter from the subarachnoid space. The direction of the catheter
introduced into the subarachnoid space cannot be predicted. In this
work, with professionals over 45 years of practice and introduction
of less than 2 cm of the catheter, a 6% incidence of paresthesia was
observed. Post dural-puncture headache (PDPH) is a commonly
reported complication of spinal anesthesia. A decrease in the size
of the puncture needle and an increasing age of the patient are
thought to reduce its incidence, but also factors such as thickness
of the dura, a thicker dura tends to retract more rapidly than thin
dura and gender of the patient, females have a higher incidence,
are to be taken into consideration [16]. In a 1999 study with a
catheter outside the needle, there were two patients with mild
post dural-puncture headache after CSA, who did not require any
invasive therapy, and two patients who received a blood-patch [11].
In another study with the same kit for CSA in 50 patients, no case of
PDPH was observed [9]. In our study with 448 patients, PDPH was
observed in only 7 patients (1.5%) of medium intensity and short
duration (3 days). Postoperative urinary retention is a common
event following surgical procedures. As criteria for inclusion in
the study, patients who had a bladder catheter were automatically
excluded from the study. Likewise, no patient received opioids
subarachnoid ally and analgesia was performed with lumbar plexus
block. In this study, only 1.7% of patients needed a urinary catheter
during the postoperative period.
Conlusion
The main advantage of CSA is the possibility to gradually inject the local anesthetic and control dispersion in the CSF, providing security and control over the needs of each patient. This objective was achieved in this study. The frequency of headache with this technique and in this age group is very low. No serious neurological complications were observed, especially cauda equina syndrome. Thus, we can say that CSA when correctly used with a catheter outside the needle is a safe technique, especially in elderly patients with hip or hip fractures. The CSA with high doses of hyperbaric anesthetics through the catheter outside the needle, poor distribution was not observed or risk of cauda equina syndrome were not observed. 17 Unfortunately this catheter was discontinued by the manufacturer and we anesthetists have lost an excellent product in our therapeutic arsenal.
References
- Hurley RJ, Lambert DH (1990) Continuous spinal anesthesia with a microcatheter technique. Preliminary experience. Anesth Analg 70(1): 97-102.
- Rigler ML, Drasner K, Krejcie TC, Yelich SJ, Scholnick FT, et al. (1991) Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg 72(3): 275-281.
- Lambert DH, Hurley RJ (1991) Cauda equina syndrome and continuous spinal anesthesia. Anesth Analg 72(6): 817-819.
- Möllmann M, Van Steenberge A, Sell A (1996) Spinocath, a new approach to continuous spinal anaesthesia. Preliminary results of a multicenter trial. Int Monitor 8: 74
- Imbelloni LE, Gouveia MA (1999) Avaliação de um novo cateter para raquianestesia contí Rev Bras Anestesiol 49(5): 315-319.
- Imbelloni LE, Beato L (2002) Comparison between spinal, combined spinal-epidural and continuous spinal anesthesias for hip surgeries in elderly patients. A retrospective study. Rev Bras Anestesiol 52(3): 316-325.
- Imbelloni LE, Gouveia MA, Cordeiro JA (2009) Continuous spinal anesthesia versus combined spinal epidural block for major orthopedic surgery: prospective randomized study. Sao Paulo Med J 127(1): 7-11.
- Imbelloni LE, Gouveia MA (2006) Continuous spinal anesthesia with Spinocath® for obstetric analgesia. Int J Obstet Anesth 15(2): 171-172.
- Elfeky MA, Stohy SM, Sabra MM, Mahareak AA, Alkumity AA, et al. (2019) Randomized comparison of continuous spinal anesthesia versus continuous epidural anesthesia in high-risk elderly patients undergoing major orthopedic lower limb surgeries. Research and Opinion in Anesthesia & Intensive Care 6(1): 72-79.
- Dean H (1907) Discussion on the relative value of inhalation and injection methods of inducing anaesthesia. Br Med J 2: 869-877.
- Möllmann M, Cord S, Holst D, Auf der Landwehr U (1999) Continuous spinal anaesthesia or continuous epidural anaesthesia for post-operative pain control after hip replacement? Eur J Anaesthesiol 16(7): 454-461.
- De Andrés J, Valía JC, Olivares A, Bellver J (1999) Continuous spinal anesthesia: a comparative study of standard microcatheter and Spinocath. Reg Anesth Pain Med 24(2): 110-116.
- Imbelloni LE, Carneiro ANG, Sobral MGC (1995) Time for cerebrospinal fluid backflow through Quincke spinal needles. Rev Bras Anestesiol 45: 155-158.
- Michaloudis D, Petrou A, Bakos P, Chatzimichali A, Kafkalaki K, et al. (2000) Continuous spinal anaesthesia/analgesia for the perioperative management of high-risk patients. Eur J Anaesthesiol 17(4): 239-247.
- López MM, Guascha E, Schiraldi R, Maggi G, Alonso E, et al. (2016) Continuous spinal anaesthesia with minimally invasivehaemodynamic monitoring for surgical hip repair in two patients with severe aortic stenosis. Rev Bras Anestesiol 66(1): 82-85.
- Imbelloni LE, Sobral MGC, Carneiro ANG (2001) Postdural puncture headache and spinal needle design. Experience in 5050 cases. Rev Bras Anestesiol 51: 43-52.
- Imbelloni LE, Gasparini Neto S, Ganem EM (2010) Continuous spinal anesthesia with high dose of local anesthetics. Rev Bras Anestesiol 60(5): 537-543.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...