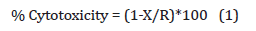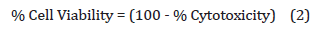
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6003
Research Article(ISSN: 2638-6003) 
Assessment of Bone Health Biomarker (ALP) after Treatment with Biofield Energy Treated DMEM in MG-63 Cells Volume 2 - Issue 4
Mahendra Kumar Trivedi1* and Snehasis Jana2
- 1Trivedi Global, Inc., Henderson, USA
- 2Trivedi Science Research Laboratory Pvt. Ltd., Bhopal, India
Received:February 07, 2019; Published: February 15, 2019
Corresponding author: Mahendra Kumar Trivedi, Trivedi Global, Inc., Henderson, USA
DOI: 10.32474/OSMOAJ.2019.02.000145
Abstract
The aim was to examine the effect of Biofield Treated DMEM on bone health in MG-63 cells. Dulbecco’s Modified Eagle’s Medium was used as a test sample in this study and divided into two parts. First part received Biofield Treatment by Mahendra Kumar Trivedi and was labeled as the one-time Biofield Energy Treated (BT-I) DMEM, while the second part received two-times Biofield Treatment and denoted as BT-II. MTT assay was studied for cell viability, which was found as more than 73.9% in MG-63 cell line, which indicated with a safe and non-toxic profile. The bone health parameter, the level of ALP was significantly (p≤0.001) increased by 42.86% in both BT-I and BT-II groups compared with the untreated DMEM group. Thus, the data suggested that the Biofield Treated DMEM significantly improved ALP level, which can be used for the management of osteoporosis, deformed bones, bone and/or joint pain, rickets, osteoma, osteomalacia, aging, hormonal imbalance, and stress.
Keywords: Biofield Energy; ALP; Osteoporosis; Osteosarcoma Cells; DMEM; Bone Health
Abbreviations: NCCAM: National Center for Complementary and Alternative Medicine; ALP: Alkaline phosphatase; MG-63: Human Bone Osteosarcoma Cells; CAM: Complementary and Alternative Medicine; FBS: Fetal Bovine Serum; DMEM: Dulbecco’s Modified Eagle’s Medium
Introduction
Bones play a major role such providing structure, protecting organs, anchoring muscles and storing calcium in the body. In addition, bones protect our brain, heart, and other major organs from any type of physical injuries. They also store calcium and phosphorous that are necessary to keep the bones strong and provide to the body when required. Long-lasting health problems in bone arises due to the imbalance between the right nutrition, exercise, body weight, medicines, etc. which results in fractures [1]. Due to this, various bone diseases developed and the most common is the osteoporosis that weaken the bone, which are more likely to break. Bone minerals, mass, tissues, collagen, hormone level, etc. play vital role in maintain the bone health. Bone-specific alkaline phosphatases (BALP), plasma membrane-bound glycoproteins important factor in bone health, which is widely used in bone health cell line studies. In addition, it is the useful biochemical marker of bone formation and is important for the bone mineralization, which indicate early differentiation and maturation of osteoblasts. BALP is very important for osteogenic activity [2]. In order to maintain good bone health, major nutrients such as calcium and vitamin D3 acts as a crucial role to maintain a healthy skeleton [3]. Other rich sources are milk and dairy products, green and yellow vegetables, soy beans, and fish are also the good sources of calcium for bone health. Calcium and vitamin D3 have significant roles as antiinflammatory, anti-arthritic, anti-osteoporosis, anti-stress, antiaging, anti-apoptotic, wound healing, anti-cancer, anti-psychotic, and anti-fibrotic roles [4-6]. MG-63 cell line is derived from juxtacortical osteosarcoma. It is extensively used for the evaluation of the bone cell growth and development of any test compounds to improve the bone health [7]. Moreover, the response of MG-63 cells after administration of 1, 25-dihydroxyvitamin D3 (1, 25(OH)2D3) has been studied as like of normal human osteoblast cells [8].
Every living organism contains subtle types of unique energy known as “Biofield Energy”. The nature of this energy is para-dimensional, infinite, and electromagnetic field that are surrounding the human body. Complementary and Alternative Medicine (CAM) therapies is widely used with significant benefits in the healthcare system. Many energy healing practices have been widely demonstrated a significant finding in healthcare system. The CAM therapies includes therapeutic touch, external qigong, yoga, Johrei, Qi Gong, Reiki, Tai Chi, polarity therapy, deep breathing, pranic healing, chiropractic/osteopathic manipulation, meditation, guided imagery, massage, homeopathy, progressive relaxation, hypnotherapy, acupressure, special diets, relaxation techniques, acupuncture, Rolfing structural integration, movement therapy, Ayurvedic medicine, healing touch, pilates, mindfulness, traditional Chinese herbs and medicines in biological systems both in vitro and in vivo [9]. Biofield Energy contain a putative bioenergy, which can be channeled by a renowned practitioner. “Biofield Energy Healing” has been proven as CAM and manifested excellent outcomes in various biological studies [10]. On the other side, National Center for Complementary and Alternative Medicine (NCCAM) well-defined that the Biofield therapies in the subcategory of Energy Therapies [11]. Biofield Treatment has been reported with significant revolution in the physicochemical properties of metals, chemicals, ceramics and polymers [12-14], improved agricultural crop yield, productivity, and quality [15,16], transformed antimicrobial characteristics [17-19], biotechnology [20,21], improved bioavailability [22-24], skin health [25,26], nutraceuticals [27,28], cancer research [29,30], human health and wellness, and bone health [31-33]. On the basis of significant results of Biofield Energy Treatment, authors planned to evaluate the effect of the Biofield on DMEM for bone health activity in MG-63 cells.
Material and Methods
Chemicals and Reagents
Penicillin and streptomycin were procured from HiMedia, India, while 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium) (MTT), Direct Red 80, and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma, USA. Rutin hydrate was purchased from TCI, Japan. Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were purchased from Life Technology, USA. All the other chemicals used in this experiment were analytical grade procured from India.
Maintenance of MG-63 in Culture Medium
Human bone osteosarcoma cell line (MG-63) was used as test system in this experiment. It was maintained in DMEM growth medium for routine culture supplemented with 10% FBS. Growth conditions were maintained at 37°C, 5%CO2, and 95% humidity and sub cultured by trypsinisation followed by splitting the cell suspension into a new flask with new medium. Three days before the start of the experiment (i.e., day -3), the growth medium of near-confluent cells was replaced with fresh phenol-free DMEM, supplemented with 10% charcoal-dextran stripped FBS (CD-FBS) and 1% penicillin-streptomycin [34].
Experimental Design
The experimental groups consisted of group 1 (G-I) with cells in untreated DMEM. Group 2 (G-II) consisted of positive control (rutin hydrate) at non-cytotoxic concentrations. Further, group 3 (G-III) and 4 (G-IV) included one-time Biofield Treated DMEM (BT-I) and two-times Biofield Treated DMEM, respectively.
Consciousness Energy Healing Treatment Strategies
The test item, DMEM medium was divided into two parts. One part each of the test item was treated with the Biofield Energy by a renowned Biofield Energy Healer, Mahendra Kumar Trivedi and coded as the Biofield Energy Treated DMEM, while the second part did not receive any sort of treatment and referred as the untreated DMEM group. This Biofield Energy Healing Treatment was provided by Mahendra Kumar Trivedi remotely for ~3 minutes. Biofield Energy Healer was located in the USA, while the test item was located in the research laboratory of Dabur Research Foundation, New Delhi, India. This Biofield Energy Treatment was administered through the Healer’s unique Energy Transmission process remotely to the test sample under laboratory conditions. Healer, in this study never visited the laboratory in person, nor had any contact with the test item. Further, the untreated DMEM was treated with a “sham” healer for comparative purposes. The “sham” healer did not have any knowledge about the Biofield Energy Treatment. After that, the Biofield Energy Treated and untreated samples were kept in similar sealed conditions for experimental study.
MTT Assay for the Assessment of Non-cytotoxic Concentration
For the evaluation on non-cytotoxic concentration of the test items (untreated and Biofield Treated DMEM) the MTT cell viability assay was performed in human bone osteosarcoma cell line (MG- 63) as per Trivedi et al. [35,36]. The percentage cytotoxicity of the test items were calculated with the help of Equation (1):
Where, X = Absorbance of treated cells; R = Absorbance of untreated cells
The percentage cell viability corresponding to each treatment was calculated with the help of Equation (2):
The concentrations ≥70% cell viability was considered as safe and non-toxic.
Alkaline Phosphatase (ALP)
Evaluation of alkaline phosphatase (ALP) activity of the untreated and Biofield Treated DMEM in human bone osteosarcoma cell line (MG-63) was conducted as per Trivedi et al. [33,34]. The level of ALP enzyme was recorded as mg/mL with respect to the untreated DMEM group.
Statistical Analysis
Data were represented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for multiple group comparison followed by post-hoc analysis by Dunnett’s test. Statistically significant values were set at the level of p≤0.05.
Results and Discussion
Determination of Non-Cytotoxic Concentration of the test items by MTT
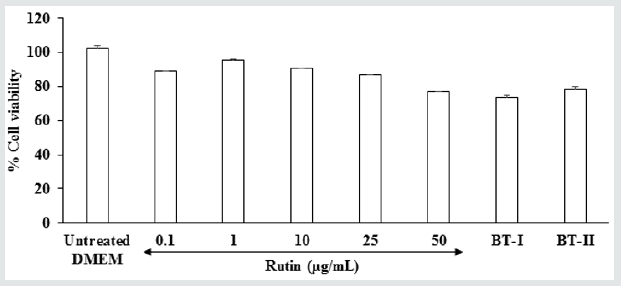
MTT assay for cell viability showed a significant improvement of cell viability among the Biofield Energy Treated test sample (DMEM) in MG-63 cells. Cell viability data in term of percentage values are shown in Figure 1. The results showed that the test sample was found to have significant cell viability with more than 73.9%. Thus, MTT cell viability data suggested that the Biofield Treated DMEM was found to be safe in MG-63 cells. Therefore, the Biofield Energy Treated DMEM was used to study the bone health parameter, alkaline phosphatase (ALP) activity in MG-63 cells (Figure 1).
Figure 1: Determination of non-cytotoxic concentration of the test item (DMEM) and the positive control (rutin hydrate) in human bone osteosarcoma cells (MG-63). BT-I: One-time Biofield Treated DMEM; BT-II: Two-time Biofield Treated DMEM.
Alkaline Phosphatase (ALP) Enzyme Activity
Figure 1: Impact of Biofield on the test items (untreated and Biofield Treated DMEM) in MG-63 cells. BT-I: One-time Biofield Treated DMEM; BT-II: Two-times Biofield Treated DMEM. Values are expressed as mean ± SD. ***p≤0.001 vs. Untreated DMEM.

The level of bone ALP was tested among different groups and compared. The Biofield Energy Treated DMEM was tested with respect to the results of ALP level on MG-63 cell line and the data are presented in Figure 2. The positive control, rutin showed a significantly (p≤0.001) increased value of ALP by 214.29%, 228.57%, and 314.29% at 0.01, 0.1, and 1μg/mL, respectively with respect to the untreated DMEM group. The one-time Biofield Treated DMEM (BT-I) and two-times Biofield Treated DMEM (BT-II) groups showed significantly (p≤0.001) increased the level of ALP by 42.86% compared with the untreated DMEM group (Figure 2). Alkaline Phosphatase (ALP), zinc metalloprotein enzyme alteration results in serious bone health diseases such as post-menopausal women, bone cancers, osteoporosis, Paget’s disease of bone, healing fracture, leukemia, acromegaly, bone growth, myelofibrosis, osteogenic sarcoma, or bone metastases, and rarely myeloma. Bone ALP is considered as one of the sensitive and reliable indicator of bone metabolism activity. From literatures, it has been mentioned that children’s have high amount of ALP isoenzymes than old age peoples [36-38]. Our experimental data suggested that the Biofield Energy Treated DMEM showed a significantly improved level of the bone ALP as compared with the untreated DMEM, which can be used as an application of various bone and age-related diseases such as osteoporosis [37]. Overall, the data suggested that The Trivedi Effect® Treated DMEM could be used to improve the level of ALP and that could be beneficial for many bon-related disorders.
Conclusion
Bone health study was conducted and the Biofield Treated DMEM was used to study the level of alkaline phosphatase (ALP) enzyme. MTT assay for cell viability was done and the data showed more than 73.9% viable cells in the tested groups, which suggest that the DMEM was safe and non-toxic. ALP, the bone health parameter was significantly (p≤0.001) increased by 42.86% in both one-time Biofield Energy Treated DMEM (BT-I) and twotimes Biofield Treated DMEM groups as compared with the untreated DMEM group. The Biofield Treated DMEM was found to have a significant impact on bone ALP level. Thus, with respect to the untreated DMEM, the Biofield Treated DMEM would be highly significant in the growth of MG-63 cells. Therefore, Biofield Treated DMEM might be a suitable alternative media for cell growth, and for the management of certain disorders like rickets, osteoporosis, bone loss and fractures, Paget’s disease, deformed bones, osteoma, osteomalacia, stress, hormonal imbalance, aging. Additionally, it might be useful to improve various physiological process those are involved in the communication from one cell to another cells, cell cycling, growth, proliferation, differentiation, neurotransmission, cardiovascular functions, hormonal balance, and skin health. It can also use in organ transplants like kidney, heart, and liver transplants, hormonal imbalance, aging, and various immune-related disorders (ulcerative colitis, dermatitis, irritable bowel syndrome, Alzheimer’s disease, hashimoto thyroiditis, asthma, multiple sclerosis, pernicious anemia, sjogren syndrome, aplastic anemia, hepatitis, graves’ disease, myasthenia gravis, dermatomyositis, diverticulitis, diabetes, atherosclerosis, Parkinson’s disease, systemic lupus erythematosus, stress, etc.).
Acknowledgement
Authors are grateful to Dabur Research Foundation, Trivedi Global, Inc., Trivedi Science, Trivedi Testimonials, and Trivedi Master Wellness for their support throughout the work.
References
- Lorincz C, Manske SL, Zernicke R (2009) Bone Health: Part 1, Nutrition. Sports Health 1(3): 253-260.
- Iba K, Takada J, Yamashita T (2004) The serum level of bone-specific alkaline phosphatase activity is associated with aortic calcification in osteoporosis patients. J Bone Miner Metab 22(6): 594-596.
- Holick MF (1996) Vitamin D and bone health. J Nutr 126(4 Suppl): 1159- 1164.
- Flynn A (2003) The role of dietary calcium in bone health. Proc Nutr Soc 62(4): 851-858.
- Cashman KD (2007) Diet, nutrition, and bone health. J Nutr 137(11 Suppl): 2507-2512.
- Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrine Rev 22(4): 477-501.
- Czekanska EM, Stoddart MJ, Richards RG, Hayes JS (2012) In search of an osteoblast cell model for in vitro research. Eur Cell Mater 24: 1-17.
- Luo XH, Liao EY (2003) Effects of estriol on the proliferation and differentiation of human osteoblastic MG-63 cells. Endocrine Res 29(3): 343-351.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8(6): 703-717.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, Kaye AD (2012) Use and acceptance of complementary and alternative medicine among the general population and medical personnel: A systematic review. Ochsner J 12(1): 45-56.
- Trivedi MK, Tallapragada RM (2008) A transcendental to changing metal powder characteristics. Met Powder Rep 63(9): 22-28, 31.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage 4: 161.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Effect of biofield energy treatment on physical and structural properties of calcium carbide and praseodymium oxide. International Journal of Materials Science and Applications 4: 390-395.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L.). Journal of Food and Nutrition Sciences 3: 245-250.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of biochemical marker-Glutathione and DNA fingerprinting of biofield energy treated Oryza sativa. American Journal of Bio Science 3: 243-248.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, eta l. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3: 129.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. J Antivir Antiretrovir 7: 83-88.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) An impact of biofield treatment: Antimycobacterial susceptibility potential using BACTEC 460/MGIT-TB System. Mycobact Dis 5: 189.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. J Microb Biochem Technol 7: 203-206.
- Nayak G, Altekar N (2015) Effect of biofield treatment on plant growth and adaptation. J Environ Health Sci 1: 1-9.
- Branton A, Jana S (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. International Journal of Clinical and Developmental Anatomy 3: 9-15.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. American Journal of Clinical and Experimental Medicine 5: 138-144.
- Branton A, Jana S (2017) Effect of The biofield energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3 [25(OH)D3 ] in rats after a single oral dose of vitamin D3 . American Journal of Pharmacology and Phytotherapy 2: 11-18.
- Kinney JP, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. American Journal of Life Sciences 5: 65-74.
- Singh J, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. American Journal of Pharmacology and Phytotherapy 2: 1-10.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) A Systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. International Journal of Bioorganic Chemistry 2: 135-145.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) Chromatographic and spectroscopic characterization of the consciousness energy healing treated Withania Somnifera (ashwagandha) root extract. European Journal of Biophysics 5: 38-47.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4: 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253-257.
- Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Influence of biofield treated vitamin D3 on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. International Journal of Biomedical Engineering and Clinical Science 4: 6-14.
- Lee AC, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). International Journal of Nutrition and Food Sciences 7: 30-38.
- Stutheit ME, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Biofield energy treated vitamin D3 : Therapeutic implication on bone health using osteoblasts cells. American Journal of Life Sciences 6: 13- 21.
- Czekanska EM, Stoddart MJ, Richards RG, Hayes JS (2012) In search of an osteoblast cell model for in vitro research. Eur Cells Mater 24: 1-17.
- ISO (2009) Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity 10993(5): 20093.
- Jesudason D, Need AG, Horowitz M, OLoughlin PD, Morris HA, et al. (2002) Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone 31(5): 626-630.
- Seeman E (2009) Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 19(3): 219-233.
- Golub EE, Boesze-Battaglia K (2007) The role of alkaline phosphatase in mineralization. Curr Opin Orthop 18(5): 444-448.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...