Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1209
Review Article(ISSN: 2644-1209) 
Traumatic Chorioretinal Rupture: Diagnosis and Treatment Alternatives Volume 2 - Issue 1
Ayse Gul Kocak Altintas*
- Ankara Ulucanlar Eye Education and Research Hospital, Turkey
Received: November 08, 2018; Published: November 16, 2018
*Corresponding author: Ayse Gul Kocak Altintas, Ankara Ulucanlar Eye Education and Research Hospital, Turkey
DOI: 10.32474/TOOAJ.2018.02.000127
Abstract
Traumatic choroidal rupture is a serious complication of blunt ocular trauma with a poor visual prognosis. Patient should be monitored closely for the development of choroidal neovascularization. Early diagnosis and prompt treatment are important for prevention of severe visual deterioration. Even there is no consensus in the literature on which treatment choice offers the best anatomic and functional results intra vitreal Anti-vascular endothelial growth factor injection gives better visual outcomes.
Keywords: Traumatic choroidal rupture; Choroidal neovascularization; Fluorescein angiography; Fundus autofluorescence; Optical coherence tomography; Anti- VGF; Recombinant tissue plasminogen
Abbreviations: RPE: Retinal Pigment Epithelium; CNV: Choroidal Neovascularization; VA: Visual Acuity; FA: Fluorescein Angiography; FAF: Fundus Autofluorescence; OCT: Optical Coherence Tomography; OCT-A: Optical Coherence Tomography Angiography; IV: İntravitreal; Anti- VGF: Anti-Vascular Endothelial Growth Factor İnjection; r-TPA: Recombinant Tissue Plasminogen Activator
Introduction
Choroidal rupture is an uncommon but a serious complication of nonpenetrating, blunt ocular trauma (TCR), also known as sclopetaria, was first described in 1854 by Von Graefe as a fullthickness break of the choroid, Bruch’s membrane, and the retinal pigment epithelium (RPE) [1-3]. TCR is more common among young men because they are more frequently affected by blunt ocular trauma by physical aggression and occupational accidents than women. Motor vehicle accidents, being hit by ball or a large hard blunt object like stone, hit with an airbag, involved in either explosions or non-explosive industrial accidents were the most common trauma etiology in TCR [2-5]. Traumatic choroidal ruptures can occur either directly at the site of impact or indirectly on the opposite side of globe. Choroidal ruptures develop in approximately 5–10% of whole patients after blunt ocular trauma and indirect sub- group makes up 80-82% of all cases with TCR. Direct choroidal ruptures occur proximity to the original injury site, mainly anterior of the equator and parallel to the ora serrata while indirect choroidal ruptures are seen more posterior, away from the site of the original impact, that develop secondary to countercoup injury against the eyeball [1-6].
Patho-Physiology of Choroidal Ruptures
The ocular globe shape undergoes biphasic changes during blunt trauma initially it has antero-posterior mechanical compression by the traumatic forces and then sudden hyperextension just after force effects diminishes. The shear force transmitted concentrically outward from the peripapillary area. The different layers of the eye are affected differently by this trauma, such as the sclera can resist these changes by its high tensile strength and retina is protected due to its high elasticity. In contrast, the middle layers of retina sclera complex they have less elastic than the retina and has less tensile strength than the sclera, to resist these forces making them most susceptible to trauma. Therefore, blunt ocular trauma cause rupture in the choroid, break in the Bruch’s membrane and the retinal pigment epithelium (RPE) [2,3,6]. Even most globes have a single rupture, nearly 1/4 of cases have multiple ruptures running concentric to the optic nerve on the way where the shear force transmitted which present a crescent shape on the temporal side of the optic disc. Nearly 2/3 of ruptures involves the macula that causes profound visual disability [1,4-7]. In early phase deep subretinal hemorrhages occurs secondary to direct injury to the choroidal vessels under the rupture area, that may cause further RPE and retina damage. Pre‐retinal hemorrhages and even vitreous hemorrhage may be induced by severe traumas, but retinal detachment seldom occurs. Retinal detachments were more associated with peripheral choroidal rupture compared with macular ruptures.
Secondary choroidal neovascularization (CNV) develops among 5- 30% of patients after choroidal rupture. CNV develops as a part of wound healing process of break in Bruch’s membrane and choroid, with increased inflammatory response and abnormal production of vascular endothelial growth factor. Membrane formation of new vessels thought to be result from an imbalance of proangiogenic and antiangiogenic cytokines that are secreted in response to cellular injury on rupture areas. The development of CNV is strongly related to length of the rupture and its distance to the fovea where the most vulnerable part of retina. CNV may cause hemorrhagic or serous macular detachment that lead to poor visual prognosis [3,4,7-9]. Initial visual acuity (VA) after TCR is usually less than 20/200 and even in most of the cases it is as low as only counting finger. Initial VA and visual recovery are mostly related with severity of injuries, such as localization of rupture, degree of commotion retinae, presence of subretinal and /or intraocular hemorrhage and retinal detachment [10,11].
Diagnostic Techniques
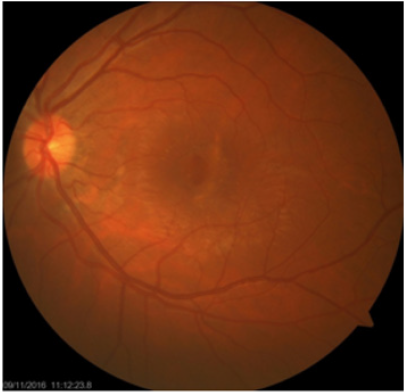
Fundus examination shows white to orange-colored crescentshaped streak at the posterior pole, generally concentric and temporal to the optic nerve which may associated with subretinal hemorrhage at the early phase of trauma then it became pigmented while the choroidal rupture scars develops. In addition, the fundus examination, different imaging tools are useful in the diagnosis and to identified the nature and severity of choroidal ruptures [1,4,6,7] (Figure 1).
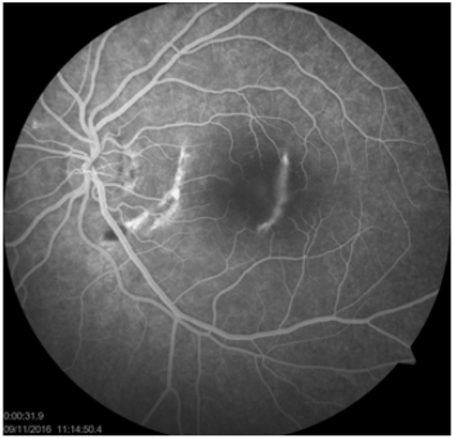
Fluorescein angiography (FA) had been used for several decades as an imaging tools in the diagnosis and study of TCR. FA shows window defect, transmission hyper fluorescence corresponding to the choroidal rupture in early phase, sub foveal hyperfluorescent shows the late leakage of dye from injured vessels or from CNM in mid phase and staining hyper fluorescence of the scarred CNM and/or choroidal rupture in healing stage. Subretinal or intraretinal hemorrhage are seen hypo fluorescence due to their blockage effect on choroidal fluorescence [3,6-8,10] Figure 2. Fundus autofluorescence (FAF) image could better delineate the pattern and the length of choroidal rupture than fundus photography; reduced FAF and surrounding increased auto fluorescent rim due to increased lipofuscin, corresponding to hyperpigmented area in the fundus photography.
Figure 2: Hyperfluorescence curvilinear window defects on Fundus fluorescein angiography reveals choroidal rupture areas.
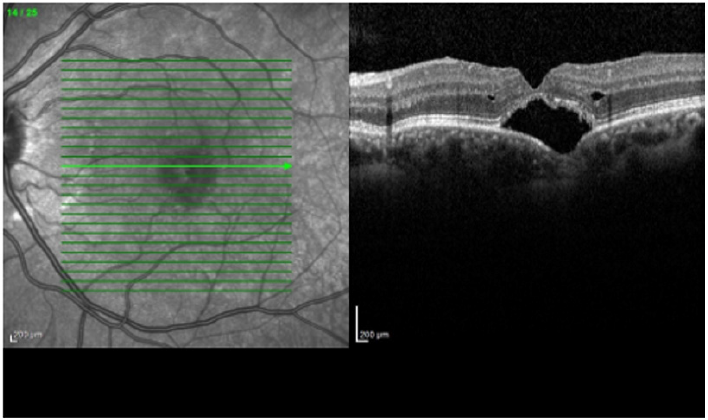
Areas of damaged RPE are more evident by reduced FAF similar to recent subretinal hemorrhage. The areas of reabsorbed hemorrhage appear with mild reduction of FAF, later on the subfoveal free‐radical, iron compound that degraded form blood cells appears as hyper auto fluorescent deposits [12,13]. Optical coherence tomography (OCT) is another useful exam method for the evaluation the integrity, length and severity of posttraumatic choriocapillaris/RPE complex and retinal lesions such as disrupted layers of the ELM and IS/OS junction (ellipsoid zone). OCT is more accurate than color photography to show detailed findings after blunt ocular trauma which also reveals the areas of retinal detachment, subretinal hemorrhage and CNV if present, as complications of TCR. RPE hyperplasia with accumulation of lipofuscin during the resolution of choroidal rupture presents as increased reflectivity in OCT, similar to increased autofluorescence [13,14] (Figure 3).
Figure 3: Optical coherence tomography (OCT) shows accumulation of subretinal fluid under the disrupted retinal pigment epithelium/Bruch membrane complex.
Optical coherence tomography angiography (OCT-A) is a recently developed medical imaging technique used to produced noninvasive high-resolution cross-sectional images of biological tissues that can add relevant information for both initial histological nature of choroidal ruptures and its follow-up. OCT-A shows hypointense appearance corresponding to breaks in the choriocapillaris plexus due to the lack of substance and hyperintense appearance as the projection of the larger choroidal vasculature underlying lesions. The presence of CNV as a hyperintense lesion and destruction of retinal vascular plexus as a hypointense area can be evaluated by OCT-A [6,13,15].
Treatment Methods
Patients with TCR should be follow up closely for the development of chorio retinal complications. The observation of peripheral retina is necessary for detection of associated retinal detachment. The most common complications from choroidal rupture is development of choroidal neovascularization and most of them occurs during the first year after injury. If choroidal neovascular membrane (CNM) develops, different treatment modalities can be applied based on the its localization. Extrafoveal membranes should be treated with laser photocoagulation but unfortunately the majority of posttraumatic CNM are sub foveal, therefore laser photocoagulation causes severe visual lose due to its more destructive effect in vast majority of patient [10].
It has been reported different treatment modalities for sub foveal CNM, including the use of photodynamic therapy, intravitreal (IV) anti-vascular endothelial growth factor injection (Anti- VGF), IV and/or systemic steroid treatment, recombinant tissue plasminogen activator (r-TPA) with or without gas injection [10,16- 20].
Photodynamic therapy with verteporfin injection in patient with TCR has certain limitations like a risk of post-treatment vision loss and high expenses [18,19]. Inflammation as a part of healing process in fibrovascular proliferation around the choroidal rupture can suppress with steroid therapy. Moon et al. [1] reported that a significant visual acuity improvement after steroid pulse therapy with methylprednisolone 500 mg intravenous for 3 days and prednisolone 30 mg oral, tapering for 11 days. The potential complications of high-dose steroids require close monitoring mainly in treatment of young children.
IV gase injection with r-TPA is an effective choice for displacement of sub macular hemorrhage for preventing toxic effects of hemosiderin comes from degenerated blood cells to the RPE and retinal cells. But r-TPA has potential risk of recurrent hemorrhage and retinal toxicity itself. IV gases injection can be performed with IV bevacizumab (Avastin, 1.25 mg/0.05 ml; Genentech, Inc., San Francisco, Calif., USA) injection as another effective alternative treatment without retinal toxicity in cases with subretinal hemorrhage [16,17]. Bevacizumab, is a humanized monoclonal antibody to vascular endothelial growth factor, has been using for treatment of sub foveal CNV secondary to different etiology such as–aged related macular degeneration, myopia, POHS (Presumed Ocular Histoplasmosis), anguloid streaks as well as traumatic choroidal rupture [20-23].
IV bevacizumab injection is an effective management to treat persistent serous retinal detachment by reducing exudation from CNM and regression of CNM itself. Chanana et al. [21] observed regression of the neovascular membrane and improvement of visual acuity after bevacizumab injection without any adverse effect. Francis and Freund et al. [22] observed photoreceptor reconstitution after IV bevacizumab injection for treatment of CNV secondary to TCR. Early IV bevacizumab injection is also could an effective and well tolerated treatment option for patients with subretinal fluid before development of CNV.
Conclusion
As a conclusion, TCRs are devastating ocular injuries, even with close monitoring and prompt treatment, most patients with TCR do not achieve final VA of 20/40 or better. Poor visual outcome is mostly associated with macular rupture and CNV formation as its complication. Unfortunately, still the management options for TCR are currently limited but intravitreal Anti-VEGF injection seems superior clinical outcome than other alternatives.
References
- Moon K, Kim KS, Kim YC (2013) A Case of Expansion of Traumatic Choroidal Rupture with Delayed-Developed Outer Retinal Changes Case Rep Ophthalmol 4(2): 70-75.
- Youssri AI, Young LH (2000) Closed-globe contusion injuries of the posterior segment. Int Ophthalmol Clin 42(3): 79-86.
- Secretan M, Sickenberg M, Zografos L, Piguet B (1998) Morphometric characteristics of traumatic choroidal ruptures associated with neovascularization. Retina 18(1): 62-66.
- Wyszynski RE, Grossniklaus HE, Frank KE (1998) Indirect choroidal rupture secondary to blunt ocular trauma. A review of eight eyes. Retina 8(4): 237-243.
- . Papakostas TD, Yonekawa Y, Wu D, Miller JB, Veldman PB, et al. (2014) Retinal detachment associated with traumatic chorioretinal rupture. Ophthalmic Surg Lasers Imaging Retina 45(5):451-455.
- Petrarch R, Saldana M (2012) Choroidal rupture and optic nerve injury with equipment designated as ‘child-safe’. BMJ Case Rep.
- Shin JY, Chung B, Na YH, Lee J, Chung H, et al. (2017) Retinal pigment epithelium wound healing after traumatic choroidal rupture. Acta Ophthalmol 95(7): 582-586.
- Takahashi M, Kinoshita S, Saito W, Kase M, Ishida S (2011) Choroidal neovascularization in a patient with blunt trauma-caused traumatic retinopathy without choroidal rupture. Graefes Arch Clin Exp Ophthalmol 249(1): 137-140
- Abri A, Binder S, Pavelka M, Tittl M, Neumuller J (2006) Choroidal neovascularization in a child with traumatic choroidal rupture: clinical and ultrastructural findings. Clin Exp Ophthalmol 34(5): 460-463.
- Ament CS, Zacks DN, Lane AM, Krzystolik M, D’Amico DJ, et al. (2006) Predictors of Visual Outcome and Choroidal Neovascular Membrane Formation After Traumatic Choroidal Rupture. Arch Ophthalmol 124(7): 957-966
- Bressler SB, Bressler NM (1998) Traumatic maculopathies. In: Shingleton B, Hersh P, Kenyon K, eds. Eye Trauma. Boston, Mass MosbyYear Book Inc pp. 190-193.
- Guerra RL, Silva IS, Marback EF, Maia Ode O, Marback RL (2014) Fundus autofluorescence in blunt ocular trauma. Arq Bras Oftalmol. 77(3): 139- 142.
- Pierro L, Giuffrè C, Rabiolo A, Gagliardi M, Arrigo A, et al. (2017) Multimodal Imaging in a Patient with Traumatic Choroidal Ruptures. Eur J Ophthalmol. 27(6): 175-178.
- Sony P, Venkatesh P, Gadaginamath S, Garg SP (2006) Optical coherence tomography findings in commotio retina. Clin Experiment Ophthalmol 34: 621-623.
- Perrott Reynolds R, Cann R, Cronbach N, Neo YN, Ho V, et al. (2018) The diagnostic accuracy of OCT angiography in naive and treated neovascular age-related macular degeneration: A review. Eye.
- Handwerger BA, Blodi BA, Chandra SR, Olsen TW, Stevens TS (2001) Treatment of submacular hemorrhage with low-dose intravitreal tissue plasminogen activator injection and pneumatic displacement. Arch Ophthalmol 119(1): 28-32.
- Altintas AGK, Ilhan C, Citirik M (2018) Successful treatment of an extensive retinal damage due to blunt ocular trauma New Front Ophthalmol 4(1): 1-3.
- Harissi-Dagher M, Sebag M, Gauthier, Marcil G, Labelle P, et al. (2005) Photodynamic therapy in young patients with choroidal neovascularization following traumatic choroidal rupture. Am J Ophthalmol 139(4): 726-728.
- Mennel S, Hausmann N, Meyer CH, Peter S (2005) Photodynamic therapy and indocyanine green guided feeder vessel photocoagulation of choroidal neovascularization secondary to choroid rupture after blunt trauma. Graefes Arch Clin Exp Ophthalmol 243(1): 68-71.
- Kim M, Kim JH, Seo Y, Koh HJ, Lee SC (2015) Intravitreal Bevacizumab for Traumatic Choroidal Rupture. Optom Vis Sci 92(10): 363-367.
- Chanana B, Azad RV, Kumar N (2009) Intravitreal bevacizumab for subfoveal choroidal neovascularization secondary to traumatic choroidal rupture. Eye (Lond) 23(11): 2125-2126.
- Francis JH, Freund KB (2011) Photoreceptor reconstitution correlates with visual improvement after intravitreal bevacizumab treatment of choroidal neovascularization secondary to traumatic choroidal rupture. Retina 31(2): 422-424.
- Piermarocchi S, Benetti E, Fracasso G (2011) Intravitreal bevacizumab for posttraumatic choroidal neovascularization in a child. J AAPOS 15(3): 314-316.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...