Lupine Publishers Group
Lupine Publishers
Review ArticleOpen Access
Technology Trend Update And Accommodation Mechanisms of Presbyopia Surgical Treatment: A Comprehensive Review Volume 3 - Issue 5
Jui-Teng Lin*
- New Photon Corp. New Taipei City, Taiwan ROC
Received:February 2, 2023; Published:February 10, 2023
Corresponding author: Jui-Teng Lin, New Photon Corp. New Taipei City, Taiwan ROC
DOI: 10.32474/TOOAJ.2023.03.000173
Abstract
Purpose: To review the technology and principles of laser presbyopia and update the future technology trend with a proposed dual-wavelength laser system.
Methodology: The accommodation gain (AG) after a laser treatment is governed by multiple factors including: the lens front and back curvature change (or lens thickening), thickening of ciliary body and its apex, the length of anterior vitreal zonules (AVZ), posterior vitreal zonules (PVZ), cross vitreal zonules (CVZ) and the space of ciliary body and lens equation (SCL). The increase of AG and/or SCL can be achieved by softening (or ablating) the sclera to improve age-related loss in forward movement of the vitreous zonules posterior insertion zone. The measured data of accommodative response of the above elements are analyzed. Besides a review of prior publications, the future technology trend with a proposed novel strategy for improved accommodation using a dual-wavelength laser system. In addition, the action mechanism of accommodation based on the measured data will be further explored. Finally, new directions and trends are proposed.
Conclusion: The accommodation gain (AG) after a laser treatment is governed by multiple factors which increase the SCL and the forward movement of the vitreous zonules. The drawbacks of prior arts might be overcome by newer technology as shown by the new trends/directions proposed in the present article.
Keywords: Presbyopia; Accommodation; Scleral, Ciliary body, Zonules, Lasers
Introduction
Presbyopia is the ocular condition which arises 100% on patients above age 50, with a peak prevalence at the age of 52 years [1]. According to the World Health Organization (WHO), the total number of presbyopic patients in the world is about 1.3 billion, which has doubled in 2015 [2]. The treatments for presbyopia include pharma and surgical therapies. The medication therapies include [3]: (i) softening crystalline lens for recovery of natural dynamic accommodation of the ciliary body, (ii) producing miosis of the pupil to allow for expansion of depth of field (DOF), and (iii) increase corneal tissue pliability to allow for rigid contact lens for multifocal shape profile. The surgical strategies for correction of near vision loss in presbyopia including: (i) changing of the optical pathway by corneal reshaping, (ii) altering function of the accommodative mechanism of the scleral and/or ciliary tissues, and (iii) softening the lens itself. Currently, the surgical options to treat presbyopia include: bifocal or progressive spectacles, monotonal or multifocal contact lenses, corneal or intraocular surgical procedures, scleral ablation or softening. Greater details are listed in Table 1. Review articles for the treatment of presbyopia, including the mechanisms, principles and various surgical systems, have been published [4- 14], in which Lin [12-14] has recently proposed new laser systems for invasive presbyopia treatments using a single-wavelength IR diode laser. The present Review article further updates the future technology trend with a proposed novel strategy for improved accommodation using a dual-wavelength laser system. In addition, the action mechanism of accommodation based on the measured data will be further explored [13,14]. Finally, new directions and trends are proposed.
Summary of various technology for presbyopia corrections include [5,13]; SEB (scleral expansion band), SRI (scleral radial incision by knife), SEP (silicon expansion plugs), BIC (band implanted in ciliary body), LPR (laser presbyopia reversal using scleral ablation), CK (conductive keratoplasty), DTK (diode laser thermal keratoplasty), LASIK (presbyopia LASIK using monovision), AIOL (accommodative IOL). Table 1 summarizes the technologies for presbyopia corrections based on the areas of treatments of: cornea, lens, sclera and ciliary muscle [4,13].
Presbyopia Accommodation
The Mechanisms of Accommodation
Figure 1: Schematic depiction of an unaccommodated normal eye (left) and a presbyopic eye (right) [12].
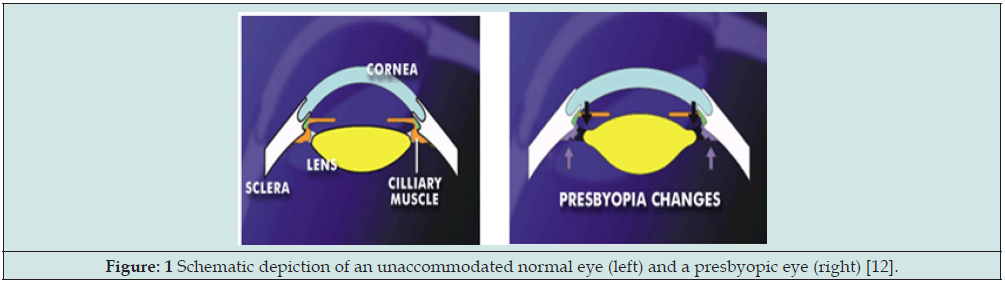
The accommodative theory, postulated by Helmholtz (1855), remains the most widely supported and cited [40-44]. As shown in Figure 1 presbyopia is due to progressive weakening or atrophy of the ciliary muscles. Accommodation is the ability to focus on near objects through controlled changes in the shape and thickness of the crystalline lens and mediated by ciliary muscle contraction [40,42]. The effectiveness of ciliary muscle contraction for lens relaxation (or accommodation) may be influenced by the combined aging factors, including lens property changes (index, size, thickness and curvature), tissue elastic changes (in sclera and ciliary) and the zonular tension change [40,42].
Figure 2: Schematic of an eye structure consists of [41,42]: cornea (11), scleral spur (12), sclera (13), ciliary body (14) and its apex (15), the anterior vitreal zonules (AVZ) (16), posterior vitreal zonules (PVZ) (17), the cross vitreal zonules (CVZ) (18a, 18b), lens(20) and iris (21), where space (CLS) (19) is the distance between the ciliary ciliary body apex (15) and the lens equator [12,40].
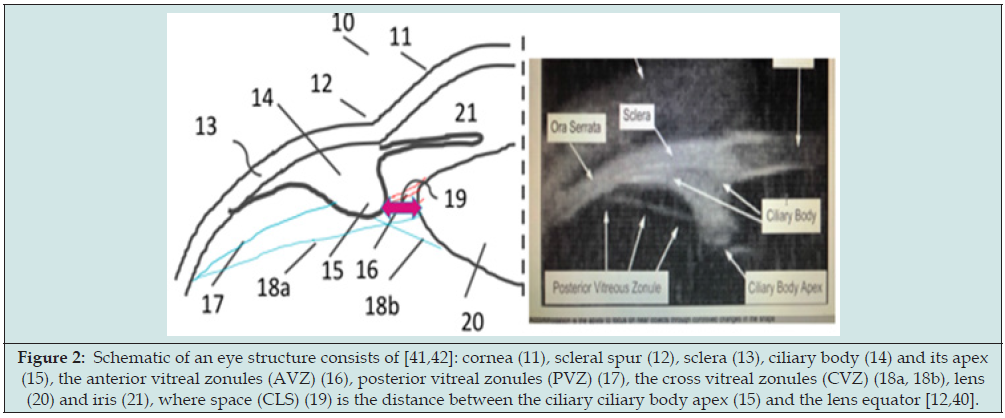
Figure 2 shows the eye structure consists of [cornea, sclera (13), ciliary body and its apex, the anterior vitreal zonules (AVZ), posterior vitreal zonules (PVZ), the cross vitreal zonules (CVZ), lens and iris, where circumlental space (CLS) is the distance between the ciliary ciliary body apex and the lens equator. Rigorous biomechanical model of the human lens and accommodation was reported with finite element modeling to show the dynamic accommodation and the changes for the key parameters were measured [40-44]. The ciliary muscle is the core tissue for the function of accommodation process. It consists of three groups of muscle fibers: longitudinal, radial and circular, having volume percentage of 12%, 33% and 55% respectively [40]. Contraction of the entire ciliary muscle as a whole pulls the anterior choroid forward, moving the apex of the ciliary processes towards the lens equator, and serves the primary function of releasing resting zonular tension at the lens equator to allow accommodation. During contraction of the ciliary muscle, the circular portion of the ciliary muscle tends to increase in thickness, whilst the radial and longitudinal portions decrease in thickness [40]. Furthermore, the lens undergoes several changes: its diameter decreases, its thickness increases, the anterior and posterior surfaces of the lens move anteriorly and posteriorly, respectively and the curvatures of the anterior and posterior surfaces of the lens increase [40,42].
It was reported [42] that during accommodation, the lens equator moved forward (anteriorly) with respect to the scleral spur by 0.48 mm in the young human eyes, by 0.45 mm in the middle-aged group, and by 0.11 mm in the older eyes. The CLS was significantly related to accommodative amplitude and declined significantly with age [42]. In the young human subjects, the unaccommodated CLS was very similar to the accommodated state. Therefore, the anterior zonules (AVZ), which extend from the region of the ciliary processes to the lens equator, were observed to be taut in the unaccommodated and accommodated states. In the older eyes, the CLS in the accommodated state was 0.15 mm narrower than in the unaccommodated state. The greater the narrowing of the CLS from the unaccommodated to the accommodated state, the lower the accommodative amplitude. The ciliary muscle apex thickening in young eyes was 0.34 mm, and was reduced to 0.15 mm in middleaged eyes and to 0.08 mm in older eyes.
In response to pilocarpine [42], the muscle apex width increased significantly with age in the unaccommodated human eyes, but not in the accommodated eyes. Thus, the thicker the resting apex width, the lower the accommodative ability. As a result, the contracted muscle apex width was unrelated to accommodative amplitude because the young muscle apex thickened substantially in response to pilocarpine, while the older muscle apex did not. During accommodation, the PVZ moved forward in a sagittal plane along the curvilinear boundary of the globe (anteriorly, toward the scleral spur) by 1.01, 0.46 and 0.03 mm in the young, middleaged, and older subjects, respectively [42]. Across the age range, the accommodative amplitude is proportional to the forward movement of the insertion zone. In the resting human eyes, the Spur-to-VZ insertion distance did not change with age. However, in the accommodated older eyes the Spur-to-VZ insertion distance tended to be longer, in comparison with the young eyes, due to the accommodative shortening of this distance in the younger eyes, but not the older eyes. Thus, neither the resting nor the accommodated Spur-to-VZ insertion distance was related to accommodative amplitude.
Accommodative ciliary muscle apex thickening was related significantly to accommodative lens thickening, lens centripetal movement and lens equator forward (anterior) movement [42]. The more the muscle apex thickened, the more the lens thickened, and the lens equator tended to move forward (anteriorly) and internally away from the sclera toward the optical axis of the eye, so that the overall movement was antero-inwardly during accommodation. The more the vitreous zonules insertion zone moved forward, the more the lens equator moved forward, and the more the lens thickened during accommodation. Based on above data of Croft et al proposed a model from the stepwise regression:
Accommodation= 17:62 + 20:75 x (Lens thickening) - 20:39 x(ATR apex thickness)
The calculation of the accommodative change is dependent upon the position of the PVZ, and the posterior restriction of the insertion zone’s movement during accommodation may dampen accommodative lens thickening. Therefore, another formula was proposed as [42]:
Lens Thickening= 0.56 - 0.10xAge
- 1,29x accommodative change in PVZ-C length
+ 0.3x (accommodative change in PVZ length).
Optically, it is the lens shape change to increase the refractive power of the eye and accommodation occurs. Therefore, lens is the prime component for the loss of accommodation with age. Accommodative lens thickening and the resting ciliary muscle apex thickness explained accommodative amplitude slightly better than age alone. Croft et al [42] also demonstrated that the vitreous zonules and its posterior insertion zone have a role in accommodation and presbyopia in the human eye. During accommodation, the lens equator moves forward and inward, and this movement is reduced with age, possibly due to the age-related loss in accommodative forward movement of the vitreous zonules posterior insertion zone.
Formulas for Accommodative Gain
The refractive power of an eye power is given by D=1000n/F (D in diopter, F in mm), which is determined by the corneal power (D’) and lens power (D) as follows [12]
DT = D’+ D – S(DD’)/(1000n1)+1000D’(n4-n1)T/(n4R’) (1.a)
D’ = 1000 [(n3-1)/r – (n3-n1)/r’] + bt, (1.b)
D = 1000 [(n4-n1)/R - (n4-n2)/R’] -aT, (1.c)
Figure 3: Schematics of an accommodating eye showing the anterior shift of the lens (with decreasing distance, S), and its surface curvatures, R1 and R2 (becoming more curved) [12].
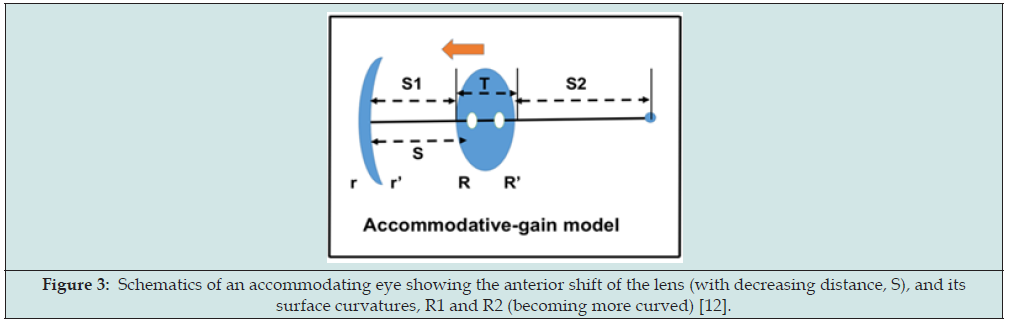
where nj (j=1, 2, 3, 4) are the refractive index for the aqueous, vitreous, cornea, and lens, respectively, the anterior and posterior radius of curvatures (in the unit of mm) of the cornea and lens are given by (r, r’) and (R, R’), respectively, noting that R’<0 for a concave surface. Finally, S is the effective anterior chamber depth, related to the anterior chamber depth (ACD). As shown in Figure 3, a 4-component theory for the accommodative gain (AG) was proposed [12]
AG = m dR + m’ dR’ + M dS1 + M’ dS2 (2)
where the AG is attributed by 4 components: front (dR) and anterior (dR’) lens curvature change, anterior chamber depth (ACD) change, dS1 and vitreous length change (dS2). The rate functions are defined in Eq. (4), with renamed notations: m=M5, m’=M6, M’=M3, and M’=M4.
By using a set of typical ocular parameters: corneal radius of curvature (r’, r2)=(7.8, 6.5) mm, lens radius of curvature (R, R’)=(10.2, 6.0) mm, corneal and lens thickness (t, T)=(0.55, 4.0) mm; and S=6.0, S1=3.5 and S2=16.0 mm, or an axial length of L=3.5 + 16 + 4 = 23.5 mm, Furthermore, for each 1.0 diopter increase of corneal and lens power, the rate functions are 1.0 and 0.66 diopter, respectively, for a typical value of effective ACD, S=6.0 mm and corneal power of 43 diopter. The conversion function translates the change of the lens power to the whole eye power, having a typical value of CF=0.62 to 0.68 [45,46].
Analysis of Measured Accommodative Gain
Based on Eq. (2), Lin [12] developed a reduced formulas for the accommodative gain (AG and AG’) due to the change of the lens anterior and posterior radius of curvature, (R, R’), given by: AG = (m + M) dR, and AG’ = (m’ + M’) dR’, respectively. For typical value of m=0.53, and M=1.35, we obtain AG=1.88 dR, that is the rate function of AG due to lens anterior curvature increase (or myopic shift) defined by RA=-dR/d(AG)=11/1.88=-0.53 (mm/D), Similarly, For typical value of m’=1.48, and M’=-2.67 (D/mm), AG’=4.15dR’, we obtain another rate function RA’= dR’/d(AG’)=1/4.15=-0.24. We note that AG is more sensitive to dR’ and dR, because typical value of R’=6.0 mm, much smaller than R=10.2 mm. These theoretically predicted values (for dR and dR’) are very close to (within 10%) the measured data of Martinez Enriquez et al. [47]. Figure 4 shows the measured average rate functions (or slopes) (shown in bars and dashed curves), and theoretical curves (in solid red curves). Similar data were also reported by the modelling of Cabeza Gil et al [40,41].
It was reported by Herek [46] that in non-presbyopic eyes, the length of PVZ changes from 4.6 mm in the un-accommodative state (UAS) to 3.6 mm in the accommodative state (AS) for a net change of 1.0 mm. In comparison, PVZ mobility is substantially reduced in presbyopic eyes: the PVZ length changes from 4.6 mm in the UAS to 4.45 mm in AS. for a net change of only 0.15mm. Furthermore, the CLS is significantly smaller in presbyopic eyes compared to non-presbyopic eyes: with measured values of 0.68mm and 0.35mm (in UAS) and 0.68mm and 0.2 mm (in AS), respectively. They also reported that the mid-stroma of the sclera can be heated to approximately 60°C to increase scleral elasticity and shrink the mid-stroma within a range of 100 um to 250 um of shrinkage, and thereby increase the CLS within a range from 200 to 500 um. The inward mobility of the ciliary body can be enhanced post-treatment by approximately 250 um.
Figure 4: Measured data of accommodative response of the lens vs. various ocular parameters of Martinez-Enriquez et al. [23], shown in bars and dashed curves; and theoretical curves (in solid red curves); for the change of lens curvatures (dR and dR’), top figures; and anterior shift, d(ACD) and corneal thickness change, d(LT), low figures [47,12].
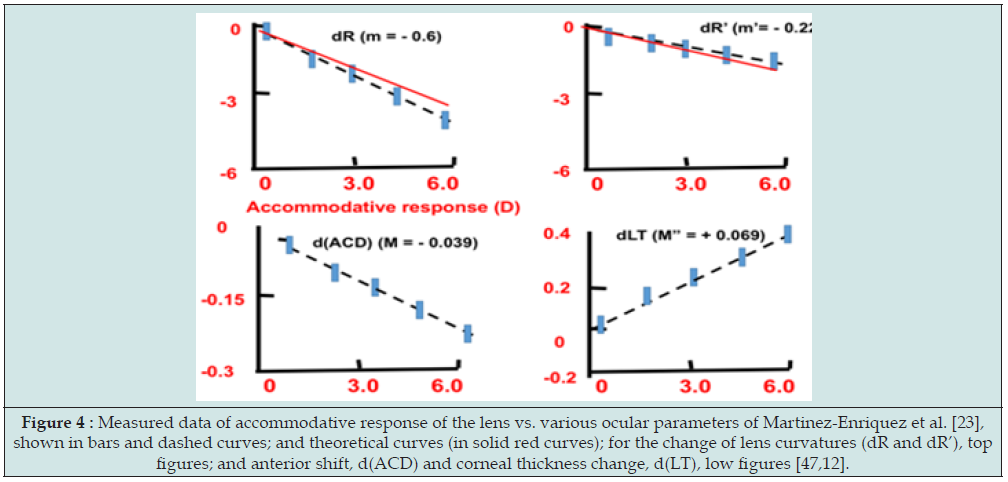
Gil [40] reported the measured and calculated change in: (i) the anterior (R) and posterior (R’) lens radius of curvature, (ii) lens thickness (TL), (iii) anterior chamber distance (ACD), (iv) anterior and posterior lens movement, and (v) lens diameter, as functions of the change in accommodation. The linear response with an increase in accommodation of 5.82 D and an increase in TL of 0.44 mm for a reduction of 0.66 mm in the ciliary muscle ring diameter, or 67.54 μm in the maximum ciliary muscle thickness (CMax). Their data are comparable with that of Ruggeri et al. [46] reported change of TL and CMax values of 0.20 mm and 72.0 μm, respectively, for an accommodation stimulus of 4 D in a 22-year-old subject. Richdale et al. [45] also reported a change of 0.38 mm and 98.41 μm, respectively for TL and Cmax, for a 5.85 D accommodative response in a 29-year-old subject.
It was proposed that accommodation may be improved by [12,46]: (i) thermal shrinkage (with temperature range of 50 0C to 70 0C) of the scleral stroma such that the space between the lens and ciliary body (CLS), or ciliary apex ring diameter (CAD) increases; or (ii)softening of the scleral stroma (with temperature range of 700C to 900C) such that the length of the posterior vitreal zonules (PVZ) increases. It was reported (US Pub. No. 2020/0000634) that in nonpresbyopic eyes, the length of PVZ changes from 4.6 mm in the unaccommodative state (UAS) to 3.6 mm in the accommodative state (AS) for a net change of 1.0 mm. In comparison, PVZ mobility is substantially reduced in presbyopic eyes: the PVZ length changes from 4.6 mm in the UAS to 4.45 mm in AS. for a net change of only 0.15mm. Furthermore, the SLC is significantly smaller in presbyopic eyes compared to non-presbyopic eyes: with measured values of 0.68mm and 0.35mm (in UAS) and 0.68mm and 0.2mm in AS, respectively. They also reported that the mid-stroma of the sclera can be heated to approximately 60° C to increase scleral elasticity and shrink the mid-stroma within a range of 100 um to 250 um of shrinkage, and thereby increase the CAD about 400 um; and the SLC within a range from 200 to 500 um. The inward mobility of the ciliary body can be enhanced post-treatment by approximately 250 um. These data may be related to our formula, A=m (dS) + m’ (dR1) + m”(dR2), where dS, dR1 and dR2 are associated with the increase of CLS and PVZ. McDonald et al reported an eye at age 53 administered by pilocarpine induced an accommodation of 4.25 diopter after scleral buckling. Lens thickness increase (dt) 0.18 mm and anterior shift (dS) 0.57 mm were measured associated with the total accommodation A=A1+A2, calculated by our theory to be A2=0.53D and A1=3.78D, where a net anterior shift dS=- 0.57+0.18=-0.39mm and change rate m=1.36 (D/mm) are used [12].
Lin and Mallo reported laser sclera ablation (LASA) procedures for presbyopia patients (age 42-60, mean 53.2) to cause a mean true accommodation of 1.96 diopter [31], without myopic-shift induced pseudo-accommodation. This was justified by no change of the far vision or corneal topography in treated eyes or comparing the pre-operative and post-operative keratometer (K) readings. We propose the two-component theory [18], A=A1+A2=aA + bA, with a=(0.1-0.4) for old eyes (age 50-60) and a=(0.5-0.7) for young eye (age 40-49) having less lens capsules rigidity. For extremely rigid eyes, lens anterior shift, A2=-mdS, becomes the only contribution, but it is limited to about (1.0-1.5) diopters. Based on our theory, accommodation is easier to achieve (for a given amount of ciliary body contraction) under the following initial ocular parameters: smaller radius of curvature of the lens or the cornea; shallower anterior chamber depth or shorter globe axial length; less rigid lens capsule and larger spacing between the lens edge and ciliary muscle. Furthermore, any power changes due to corneal surface change or axial length elongation should be excluded from the true accommodation amplitude which may be further justified by the amount of lens anterior shift and the lens radius of curvature (or thickness) changes.
A Novel Dual-Color Laser System
Prior arts of US patents for presbyopia correction including such as US Pat. Nos. 5529076, 5489299, and 5722952 of Schlacher using scleral band expansion. Prior arts of US Pat. Nos. 6,258,082 and 6,263,879 of the present authors (JT Lin), and US Pat. No. 8348932 of Hipsley et al proposed the scleral ablation by ablating lasers, such as Er: YAG (at 2.94 um) and UV laser (at 266 nm). The major drawbacks of these prior arts include invasive surgery of the eye, scleral bleeding, and complex procedures and specially the post-surgery regression of accommodation within a short period of from few months to 2 years. Furthermore, the average accommodation again is about 2.0 D, which might not be enough after regression [48].
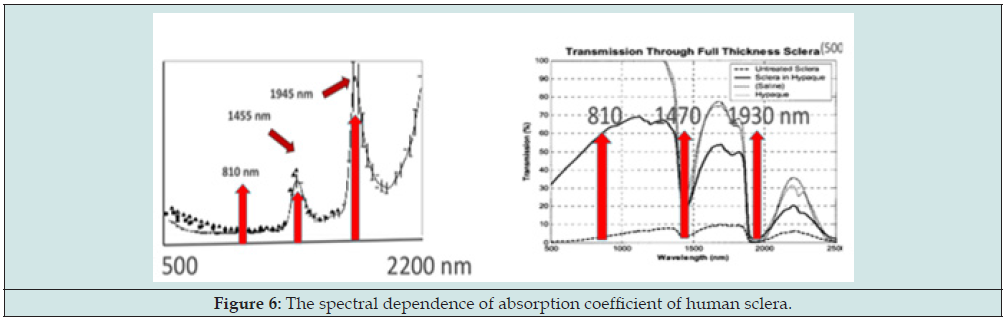
To overcome the above-described drawbacks of the prior arts, we have proposed a scleral softening system replacing the existing Er: YAG sclera ablation [12]. Another new system is proposed, as shown in Figure 5, for a dual-color laser system [37] having priority wavelength A and B, acting on the front-zone and back-zone of the sclera, respectively; where laser-A is partially transparent (>60%) to the sclera, whereas laser-B is highly absorption (>80%) to by sclera, as shown by Fig. 6 for the optical properties of human sclera [49,50], such that laser-A has a thermal penetration depth into the ciliary body (CB) (about 0.5 to 1.0 mm); and laser-B has a shallow penetration depth in the sclera (about 0.3 to 0.5 mm). Laser-A (having a wavelength about 1.4 to 2.2 um) leads to thermal shrinkage of the ciliary body such that the CLS is increased for accommodation gain which is much more effective than the prior art having a shallow penetration limited in the scleral stroma. In comparison, laser-A (having a wavelength about 800- 980 nm) leads to thermal shrinkage (and/or softening) of the sclera in the back-zone leading to the extra-space for the pulling of AVZ/PVZ during CB contraction, and the extension of PVZ-C connecting to the lens equator, such that the overall antero-inward lens movement. Accommodative ciliary muscle apex thickening was related to lens thickening and centripetal movement, but also related to the lens equator forward (anterior) movement. The more the muscle apex thickened, the more the lens thickened, and the lens equator tended to move forward (anteriorly) and internally away from the sclera toward the optical axis of the eye, so that the overall movement was antero-inwardly during accommodation. The accommodation gained in the direction as show by the “red arrows’ of Figure 5, more the vitreous zonules insertion zone moved forward, the more the lens equator moved forward, and the more the lens thickened during accommodation.
Figure 5: A novel dual-color laser system having wavelength A and B, acting on the front-zone and back-zone of the sclera, respectively. Also shown is the antero-inward movement of the ciliary body (shown by red arrows) [12, 37].
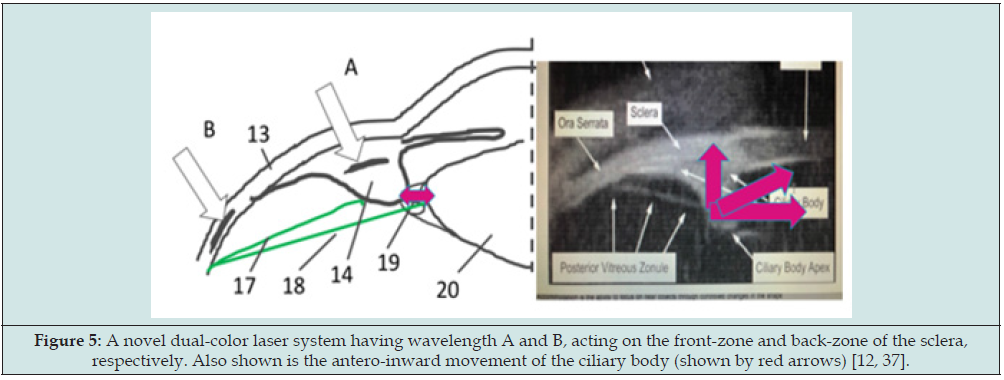
As shown in Figure 6, the laser system [37] consists of 2 output wavelength 11-A and 11-B which are coupled to 2 sets of 1x2 fiber couplers (12a and 12b) such that 4 output beams 151 to 154 are produced from the fiber ends, which are inserted to a holding base (60) contacting to the treated scleral surface. The design, as shown by Fig. 7 (right), provides laser-A and laser-B acting on the desired scleral front and back-zone, respectively, for optimal clinical outcomes projected by the proposed system.
New Trends/Directions
We note that the clinical outcomes of Ace Vision Group [32-34] are similar to that of SurgiLight [31] without any improvement, because the same Er:YAG laser and similar protocol are used by both groups based on the original US patent of Lin [26]. The major drawbacks of this method include postop regression of 0.2 to 0.5 D, after two years; too much invasive (bleedings), and the average accommodation gain (AAG) of about 2.0 diopters maybe too low if there is long term regression larger than 0.5 D. The ideal AAG would be larger than about 3.5 D, and having long term regression less than 0.5 D (for at least 5 years). In addition, the procedure time should be reduced to about 10 minutes per eye and having minimum ablation or bleeding. Based on these requirements, Lin has proposed various new methods using different lasers, and under improved protocols (US patents pending). The new directions and trends should also explore the following key issues including:
a. A minimally invasive procedure without ablating the conjunctiva or sclera tissues;
b. One system with dual-function can improve both near and far vision, or correction of hyperopia and presbyopia.
c. One system with dual-function of glaucoma and presbyopia treatments.
d. Improve the fiber coupling devices for faster procedures.
e. Higher average initial accommodation gain (AAG) > 3.5 D, such that long term regression of 0.5 D is allowed (up to at least 5 years), where the improved AG may be achieved via a novel dual-color laser systems proposed by Lin (US pat pending).
f. Understanding the “exact theories” of accommodation via more measurements such that efficacy can be improved accordingly, including the roles and changes of lens thickness and curvatures, each of the key elements of AVZ, PVZ and PVZ-C,
g. Analytic formulas to predict the clinical outcomes and the AG dependence on the eye conditions (age, lens rigidity, corneal and lens surface curvature, axial length), treated area and thickness, etc.
h. Combining pharma (medication) and surgical therapies for both short- and long-term outcomes stability.
Conclusion
The accommodation gain (AG) after a laser treatment is governed by multiple factors including: the lens front and back curvature change (or lens thickening), thickening of ciliary body and its apex, the length change of anterior vitreal zonules (AVZ), posterior vitreal zonules (PVZ), cross vitreal zonules (CVZ) and the space of ciliary body and lens equation (SCL). The increase of AG and/or SCL can be achieved by softening (or ablating) the sclera to improve age-related loss in forward movement of the vitreous zonules posterior insertion zone. The drawbacks of prior arts might be overcome by newer technology as shown by the new trends/ directions proposed in the present article.
Competing Interests
The author is the CEO of New Photon Corp. and has financial interest.
References
- Duane A (1922) Studies in monocular and binocular accommodation with their clinical applications. Am J Ophthalmol 5(11): 865-877.
- [Markets Business Insider. com. Grand View Research.
- Grzybowski A, Ruamviboonsuk V (2022) Pharmacological Treatment in Presbyopia. J Clin Med 11(5): 1385.
- Torricelli AA, Junior JB, Santhiago MR, Bechara SJ (2012) Surgical management of presbyopia. Clin Ophthalmol 6 :1459-1466.
- Therese MJ, Vargas V, Alio JL (2018) Correction of presbyopia: An integrated update for the practical surgeon. Taiwan J Ophthalmol 8(3): 121‐140.
- Baitch L (2020) Presbyopia Treatment: Current and Future Options. Review Cornea & Contact Lenses, March 15.
- Kaiti R, Kafle R, Shyangbo (2020) Presbyopia and Recent Advances in it’s Management-A Review”. EC Ophthalmology 11: 10.
- Chang D, Waring GO, Hom M, Barnett M (2021) Presbyopia Treatments by Mechanism of Action: A new classification system based on a review of the literatures. Clinical Ophthalmology 15: 3733-3745.
- Lin JT (1995) Critical review on refractive surgical lasers. Optical Engineering 668-675.
- Lin JT (2017) Progress of the 30-year laser vision technology. J Ophthalmol Clinical Res 3: 1-4.
- Lin JT (2002) Presbyopia corrections: Laser vs. Non-laser. In: Mastering the Presbyopic Surgical Lens and Phakic IOLs, Ed. Garg A, Lin JT et al, Jaypee Brother (New Delhi, 2002) pp. 219-234.
- Lin JT (2021)Analysis of Accommodation Gain of Presbyopia Eye after Laser Ablation (or Shrinkage) of Sclera via Lens Reshaping and Lens Anterior Shift. Ophthalmology Research, International Journal 15: 13-22.
- Lin JT 2021Principles of Accommodation and Technology update of Presbyopia Correction using IR and UV lasers. Ophthalmology Research, International Journal 14: 17-28.
- Lin JT (2022) Efficacy theory and proposed protocol for presbyopia correction using New Non-invasive Scleral Softening by Infrared Diode Lasers. Ophthalmology Research, International Journal 16: 24-36.
- Miranda D, Krueger RR (2004) Monovision laser in situ keratomileusis for pre-presbyopic and presbyopic patients. J Refract Surg 20(4): 325-328.
- Vargas‐Fragoso V, Alió JL (2017) Corneal compensation of presbyopia: PresbyLASIK: An updated review. Eye Vis Lond 4: 11.
- Alio JL, Amparo F, Ortiz D, Moreno L (2009) Corneal multifocality with excimer laser for presbyopia correction. Curr Opin Ophthalmol 20(4): 264-271.
- Santhiago MR, Barbosa FL, Agrawal V, Binder PS, Christie B, Wilson SE (2012) Short-term cell death and inflammation after intracorneal inlay implantation in rabbits. J Refract Surg 28(2): 144-149.
- Ruiz LA, Cepeda LM, Fuentes VC (2009) Intrastromal correction of presbyopia using a femtosecond laser system. J Refract Surg 25(10): 847-854.
- Holzer MP, Mannsfeld A, Ehmer A, Auffarth GU ( 2009) Early outcomes of INTRACOR femtosecond laser treatment for presbyopia. J Refract Surg 25(10): 855-861.
- Zhang F, Sugar A, Jacobsen G, Collins M (2011) Visual function and spectacle independence after cataract surgery: bilateral diffractive multifocal intraocular lenses versus monovision pseudophakia. J Cataract Refract Surg 37(5): 853-858.
- Patel S, Alio JL, Feinbaum C (2008) Comparison of Acri. Smart multifocal IOL, crystalens AT-45 accommodative IOL, and Technovision presbyLASIK for correcting presbyopia. J Refract Surg 24(3): 294-299.
- Thornton SP (1997) Anterior ciliary sclerotomy (ACS), a procedure to reverse presbyopia. In: Sher NA, ed. Surgery for Hyperopia and Presbyopia. Baltimore: Williams & Wilkins : 33-36.
- Fukasaku H, Marron JA 2001) Anterior ciliary sclerotomy with silicone expansion plug implantation: effect on presbyopia and intraocular pressure. Int Ophthalmol Clin 41(2): 133-141.
- Schachar RA (2001) Theoretical basis for the scleral expansion band procedure for surgical reversal of presbyopia [SRP]. Compr Ther 27(1): 39‐46.
- Lin JT. US Pats: 6824540 (2000); 6745775 (2004); RE40184 (2008).
- Lin JT, Kadambi V (2002)The new mechanism of laser presbyopia reversal and accommodation. In Agrawal S (ed.). Presbyopia: A Surgical Textbook. Thorofare, NJ: SLACK, Chapt. 6 and Chapt 13.
- Lin JT. Kadambi V, Mallo O (2003) Laser presbyopia reversal (LAPR) using laser scleral ablation. Chinese J Opt & Ophth 5: 133-135.
- Lin JT (2004) The two-component theory of accommodation after laser treatment of presbyopia. Chinese J Opt & Ophth 6: 87-91.
- Lin JT (2004) Criteria for true accommodation and pseudo-accommodation caused by axial length elongation. J. Refract Surg 20(4): 397-398.
- Lin JT, Mallo O (2003) Treatment of presbyopia by infrared laser radial sclerectomy. J Refract Surg 19(4): 465-467.
- Hipsley A, Ma DH, Sun CC, Jackson MA, Goldberg D, Hall B (2017) Visual outcomes 24 months after LaserACE. Eye Vis (Lond) 4: 15.
- Hipsley A, Hall B, Rocha KM (2018) Long-term visual outcomes of laser anterior ciliary excision. Am J Ophthalmol Case Rep 10: 38-47.
- Ting DSJ, Liu YC, Price ER (2022) Improvement in accommodation and dynamic range of focus after laser scleral microporation: A potential treatment for presbyopia. Transl Vis Sci Technol 11(12): 2.
- Lin JT (2022) Accommodative Gain in Presbyopic Eye Using a New Procedure of Laser Scleral Softening (LSS): Part-II. Formulas for Volume Efficacy. Ophthalmology Research, International Journal 16(4): 37-46.
- Lin JT (2022) Efficacy theory and proposed protocol for presbyopia correction using New Non-invasive Scleral Softening by Infrared Diode Lasers. Ophthalmology Research, International Journal. 16 24-36.
- Lin JT. (2023) US Pat. Pending.
- Gualdi L, Gualdi F, Rusciano D (2017) Ciliary muscle electrostimulation to restore accommodation in patients with early presbyopia: Preliminary results. J Refract Surg 33(9): 578-583.
- Lin JT and Martin H. US Pat.
- Cabeza-Gil I, Grasa J, Calvo B A (2021) validated finite element model to reproduce Helmholtz’s theory of accommodation: a powerful tool to investigate presbyopia. Ophthalmic Physiol Opt 41(6): 1241-1253.
- Cabeza-Gil I, Grasa J, Calvo B (2021) A numerical investigation of changes in lens shape during accommodation. Sci Rep 11: 9639.
- Croft MA, McDonald JP, Katz A, Lin TL, Lütjen-Drecoll E, et al. (2013) Extralenticular and lenticular aspects of accommodation and presbyopia in human versus monkey eyes. Invest Ophthalmol Vis Sci 54(7): 5035-5048.
- Croft MA, Nork TM, McDonald JP, Katz A, Lutjen-Drecoll E, et al. (2013) Accommodative movements of the vitreous membrane, choroid, and sclera in young and presbyopic human and nonhuman primate eyes. Invest Ophthalmol Vis Sci 54: 5049-5058.
- Croft MA, Heatley G, McDonald JP, Katz A, Kaufman PL (2015) Accommodative movements of the lens/capsule and the strand that extends between the posterior vitreous zonule insertion zone and the lens equator, in relation to the vitreous face and aging. Ophthalmic Physiol Opt 36(1): 21-32.
- Richdale K, Sinnott LT, Bullimore MA, et al. (2013) Quantification of age- related and per diopter accommodative changes of the lens and ciliary muscle in the emmetropic human eye. Invest Ophthalmol Vis Sci. 54: 1095-1105.
- Ruggeri M, de Freitas C, Williams S, Nilufer Yesilirmak, Karam Alawa, et al. (2016) Quantification of the ciliary muscle and crystalline lens interaction during accommodation with synchronous OCT imaging. Biomed Opt Express 7(4):1351-1364.
- Martinez-Enriquez E, Perez-Merino P, Velasco-Ocana M, Marcos S (2017) OCT-based full crystalline lens shape change during accommodation in vivo, Biomed Opt Express 8(2): 918-932.
- Herek S, et al. (2018) China Patent CN 105307586.
- Sacks ZS, Kurtz RM, Juhasz T and Mourau GA. (2002) High precision subsurface photodisruption in human sclera,” J. Biomed. Opt. 7(3), 442-
- Bashkatov AN, Genina EA, Kochubey VI, Tuchin VV (2010) Optical properties of human sclera in spectral range 370-2500 nm. Optics and Spectroscopy 109(2): 97-204.