Lupine Publishers Group
Lupine Publishers
Case ReportOpen Access
Posterior Polymorphous Corneal Dystrophy in A Patient with A Novel ZEB1 Gene Mutation Volume 3 - Issue 4
Eva Fernández Gutiérrez1,*, Elena Vallespín2, Pedro Fernández Pérez2, Laura García Fernández3, Mario Solis3 and Ana Boto de los Bueis1
- 1Department of Ophthalmology, La Paz University Hospital, Spain
- 2Department of Molecular Ophthalmology, Medical and Molecular Genetics Institute (INGEMM) IdiPaz, CIBERER, La Paz UniversityHospital, 28046 Madrid, Spain
- 3Department of Clinical Bioinformatics, Medical and Molecular Genetics Institute (INGEMM) IdiPaz, CIBERER, La Paz University Hospital,Spain
Received:February 12, 2022; Published:February 22, 2022
Corresponding author: Eva Fernández Gutiérrez, Department of Ophthalmology, La Paz University Hospital, 28046 Madrid, Spain
DOI: 10.32474/TOOAJ.2022.03.000167
Abstract
Objective: To describe an unusual presentation of posterior polymorphous corneal dystrophy (PPCD) and to report a previously unknown genetic alteration in the ZEB1 gene.
Methods: Biomicroscopy, confocal microscopy and genetic analysis were performed on the index patient and his first-degree relative.
Results: We present the case of a 64-year-old woman diagnosed with keratoconus who was referred for a corneal endothelium study. The patient presented endothelial lesions in both eyes suggestive of PPCD, corectopia and iridocorneal endothelial synechiae in the right eye and intrastromal segments in the left eye. The endothelial count was 825 in the right eye and 1361 in the left eye, with typical PPCD lesions visible on specular and confocal microscopy. In the genetic analysis by nucleotide sequencing, a heterozygous c.1A> C (p.Met1Leu) mutation was found in the ZEB1 gene (TCF8).
Conclusions: The PPCD3 subtype is associated with corneal ectasia, and both can appear due to a pathogenic mutation in the ZEB1 gene. However, our patient had a previously unreported mutation in the ZEB1 gene, which mediates the transition between cell lines and provides a pathogenic explanation for the epithelialization of the corneal endothelium, a characteristic of PPC.
Keywords:Posterior Polymorphous Corneal Dystrophy; Keratoconus; Iridocorneal Endothelial Syndrome; ZEB1; Confocal microscopy
Introduction
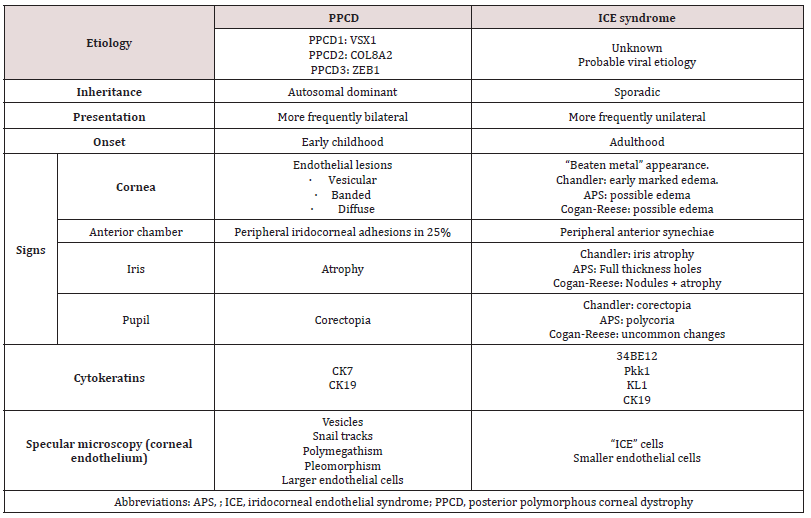
Posterior polymorphic corneal dystrophy (PPCD) affects the Descemet membrane and the corneal endothelium, occurs more frequently bilaterally and asymmetrically and has mainly autosomal dominant inheritance [1-3]. There are unilateral isolated cases with a similar phenotype but without the inheritance pattern [1]. The main endothelial corneal signs of PPCD are greyish lesions of varying shape and size, as well as vesicles and rails. In rare cases, PPCD can be accompanied by iridocorneal peripheral adhesions, iris atrophy and corectopia. Over the years, glaucoma can appear due to abnormal endothelium growth over the angle [1-3]. Pathologically, there is a proliferation of cells expressing cytokeratins CK7 and CK19 typical of epithelial lineage cells that replace the hexagonal endothelial cells, with associated secondary abnormalities in the Descemet membrane [1,4]. The biomicroscopy findings are similar to those found in iridocorneal endothelial syndrome (ICE), which can also present endothelial abnormalities, synechiae and corectopia [1,5]. Unlike PPCD, the most common form of endothelial involvement is the “beaten metal” appearance. Although both entities are characterized by endothelial epithelialization, and their cells share cytokeratin 17 typical of epithelial cells, ICE is not an inherited disease and is exceptionally bilateral (Table 1) [4,5]. The specular microscopy characteristic of PPCD are the endothelial rails, also known as “snail tracks”, which are dark areas in the form of a band that encloses a number of smaller and clear cells. The areas have irregular scalloped edges visible by slit-lamp examination. A certain degree of polymegathism and pleomorphism can also be found. However, ICE syndrome is characterized by ICE cells, which are considered pathognomonic and are characterized by larger pleomorphic cells with hyperreflective nuclei and cell borders compared with the cell surface (“light-dark inversion”) [1-6]. PPCD is a genetically heterogeneous disease with extremely variable expression in which three genes have been identified: VSX1 (20p11.21), COL8A2 (1p34.2-p32.3), and ZEB1 (10p11.22) [2,3]. We present the case of a patient with a rare biomicroscopic manifestation of PPCD, associated with keratoconus, in whom the mutation c.1A> C (p.Met1Leu) was detected for the first time in heterozygosity in the ZEB1 gene (TCF8).
Table 1: The differential diagnosis of corneal posterior polymorphic dystrophy and iridocorneal endothelial syndrome.
Clinical Case
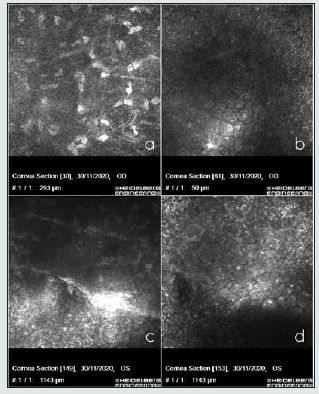
A 64-year-old woman was diagnosed with keratoconus in both eyes in October 2009 and was implanted intrastromal rings in the left eye in January 2013. She had no known systemic diseases, and her only son (aged 36 years) presented no systemic or ocular disease. In the examination, her uncorrected visual acuity was 0.5 (+2) in the right eye and 0.5 (+1) in the left eye, improving to 0.63 in the right eye with pinhole and showing no improvement in the left eye. Refractometry showed + 0.50-1.00 64º in the right eye and -0.25 -0.75 133º in the left eye. Under biomicroscopy, the right eye showed focal opacification in the inferior temporal and superior temporal corneal endothelium, anterior iridoendothelial synechiae from 4 to 6 o’clock, as well as superonasal corectopia. In the left eye, an intrastromal segment was observed at 70% depth. The corneal endothelium presented with rails in the paracentral area, together with patchy endothelial opacification. The left iris showed no abnormalities (Figures 1a-d). The lens and fundoscopy results were normal for both eyes. Intraocular pressure was 12 in the right eye and 11 in the left eye. The topography (Pentacam, Oculus) revealed a K1 (flattest meridian) of 46.6 diopters (D), a K2 (steepest meridian) of 47.3 D at 93.4º, a pachymetry apex of 557 microns and a posterior elevation of +28 microns in the right eye. The left eye had a K1 of 48.0 D, a K2 of 48.7 D at 105.4º, pachymetry of 562 microns and a posterior elevation of +38 microns which were considered a right posterior keratoconus and a left keratoconus. The specular microscopy study (NIDEX CEM 530) showed a decreased cellular endothelial count, polymegathism, and polymorphism of the endothelial cells and a number of vesicles . The Heidelberg confocal microscopy study (Heidelberg Engineering GmbH, Dossenheim, Germany) revealed keratocytes in the posterior stroma with spindle nuclei and polymorphism, polymegathism, and giant endothelial cells, with a number of nucleated cells in that lay in both eyes. In the left eye, we observed a hyporeflective crater-shaped lesion and a curvilinear, hyperreflective band lesion (Figures 2 a-d ). A genetic analysis of 6 genes associated with PPCD, included in a panel of 298 genes related to ophthalmological diseases, was conducted using the massive sequencing technique, which found the mutation c.1A> C (p.Met1Leu) in heterozygosity in the ZEB1 gene. We proposed an ophthalmological and genetic study of the patient’s descendants. The results of the biomicroscopy, specular microscopy, and genetic analysis of the proband’s 36-year-old male child were normal.
Figure 1: Biomicroscopy showing mild eccentricity of the pupil towards the upper nasal sector of the right eye (A), inferior intrastromal segment of the left eye (B), and inferior corneal endothelial opacification of the right eye(C), polymorphic grayish endothelial opacifications at different corneal locations and central endothelial rails of the left eye (D).
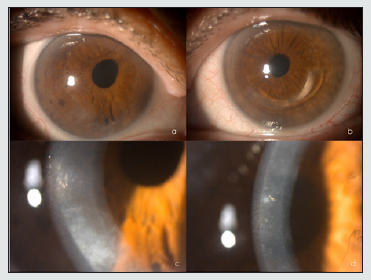
Figure 2: In vivo confocal microscopy showing posterior stromal keratocytes with elongated spindle-shaped nuclei in the right eye (A), polymorphism, polymegathism, giant endothelial cells with some nucleated cells in the right eye (B), curvilinear, hyperreflective banded lesion in the left eye surrounded by hyperreflective keratocytes(C) and hyporeflective vesicular lesions, in the form of a crater with hyperreflective deposits around the lesions in the left eye (E).
Discussion
We present the clinical case of a woman with PPCD and keratoconus, with a previously undescribed mutation in the ZEB1 gene. PPCD is inherited in an autosomal dominant form and is genetically heterogeneous, with mutations identified in 3 loci: The chromosome 20p (20p 11.21) VSX1 gene mutation is characteristic of the PPCD1 subtype, the COL8A2 gene (1p34.2-p32. 3) mutation is characteristic of the PPCD2 subtype, and the ZEB1 gene mutation is characteristic of the PPCD3 subtype [2,3]. PPCD3 is often associated with corneal steepening [7,8]. The ZEB1 gene or zinc-finger homeodomain transcription factor 8 is the gene that was altered in our patient. ZEB1 mutations have been systemically linked with Alport syndrome and the progression of certain tumors. In ophthalmological cases, the gene is associated with PPCD, Fuchs’ endothelial corneal dystrophy and keratoconus [9-12]. In contrast, individuals have also been identified with this pathogenic mutation in the ZEB1 gene who do not experience corneal disease. A 2013 study confirmed the genotype-phenotype correlation, such that mutations of the missense type in the ZEB1 protein resulted in Fuchs’ endothelial dystrophy and keratoconus, while the nonsense mutations of ZEB1 produced a Stop codon, truncating the protein, causing PPCD3 [9]. The ZEB1 gene encodes a transcription factor involved in mediating epithelial and endothelial cell lineage transitions and is crucial during embryogenesis, in the development of neural crest cell-derived structures such as the corneal endothelium [12]. The ZEB1 transcription factor is responsible for regulating the epithelial-mesenchymal transition, which involves the transformation of non-motile epithelial cells into cells with a mesenchymal phenotype that have the ability to migrate. ZEB1 induces the epithelial-mesenchymal transition by suppressing the expression of specific epithelial factors such as E-cadherin and is responsible for the reverse transition from mesenchyme to epithelium [10-12].
As a consequence of the ZEB1 mutation, several epithelial-like features are observed in the corneal endothelium of PPCD, including a stratified organization, desmosomal intracellular junctions, and expression of an epithelial-like profile, including increased expression of keratins 7 and 19 [11] and cadherins (e.g., CDH1) and decreased expression of CDH2 associated with the epithelium [12]. Mutations in the ZEB1 gene could therefore pathogenetically explain the epithelial transformation of endothelium in PPCD. In our patient, the isolated mutation was c.1A> C (p.Met1Leu) in heterozygosity in the ZEB1 gene, a mutation not previously described in the literature or databases. There has been only one patient with a pathogenic mutation described in the same nucleotide, but the change is a guanine instead of a cytosine: c.1A>G (p.Met1Val) [13]. In our patient’s case, the mutation is c.1A>C (p.Met1Leu). Therefore, the resulting amino acid in the mutation is different. The detected variant has not been previously identified in other patients. Due to its characteristics, however, the variant is considered likely to be pathogenic and, therefore, associated with endothelial dystrophy in our patient. This genetic disorder is transmitted following an autosomal dominant inheritance pattern, meaning that the proband’s children have a 50% probability of inheriting the pattern. However, these genetic disorders present variable expression, and not all individuals with the disorder develop the same symptoms, the same severity, or the same clinical progression.
References
- Weiss J, Møller H, Lisch W (2008) La clasificación IC3D de las distrofias corneales. Córnea 27(2): 43-83.
- Lin ZN, Chen J, Cui HP (2016) Characteristics of corneal dystrophies: a review from clinical, histological and genetic perspectives. Int J Ophthalmol 9(6): 904-913.
- Lam H, Wiggs J, Jurkunas U (2010) Unusual presentation of presumed posterior polymorphous dystrophy associated with iris heterochromia, band keratopathy, and keratoconus. Cornea 29(10): 1180-1185.
- Jirsova K, Merjava S, Martincova R (2007) Immunohistochemical characterization of cytokeratins in the abnormal corneal endothelium of posterior polymorphous corneal dystrophy patients. Experimental eye research 84(4): 680-686.
- Silva L, Najafi A, Suwan Y (2018) The iridocorneal endothelial syndrome. Survey of ophthalmology 63(5): 665-676.
- Laganowski HC, Sherrard ES, Muir MG (1991) Distinguishing features of the iridocorneal endothelial syndrome and posterior polymorphous dystrophy: value of endothelial specular microscopy. British journal of ophthalmology 75(4): 212-216.
- Raber I M, Fintelmann R, Chhabra S, (2011) Posterior polymorphous dystrophy associated with nonkeratoconic steep corneal curvatures. Cornea 30(10): 1120-1124.
- Aldave A J, Ann L B, Frausto R F (2013) Classification of posterior polymorphous corneal dystrophy as a corneal ectatic disorder following confirmation of associated significant corneal steepening. JAMA ophthalmology 131(12): 1583-1590.
- Lechner J, Dash D P, Muszynska D (2013) Mutational spectrum of the ZEB1 gene in corneal dystrophies supports a genotype–phenotype correlation. Invest Ophthalmol Vis Sci 54(5): 3215-3223.
- Chung D W D, Frausto R F, Ann L B (2014) Functional impact of ZEB1 mutations associated with posterior polymorphous and Fuchs' endothelial corneal dystrophies. Invest Ophthalmol Vis Sci 55(10): 6159-6166.
- Liskova P, Evans CJ, Davidson AE (2016) Heterozygous deletions at the ZEB1 locus verify haploinsufficiency as the mechanism of disease for posterior polymorphous corneal dystrophy type 3. European Journal of Human Genetics 24(7): 985-991.
- Frausto RF, Chung DD, Boere P M (2019) ZEB1 insufficiency causes corneal endothelial cell state transition and altered cellular processing. PloS one 14(6): e0218279.
- Dudakova L, Evans C J, Pontikos N, Hafford Tear N J, Malinka F, et al. (2019) The utility of massively parallel sequencing for posterior polymorphous corneal dystrophy type 3 molecular diagnosis. Exp Eye Res 182: 160-166.




