Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1209
Mini Review(ISSN: 2644-1209) 
Pachychoroid Neovasculopathy Within The Spectrum of Pachychoroid Disease Volume 1 - Issue 3
Di Carlo E1,2* and Augustin AJ1
- 1Department of Ophthalmology, Staedtisches Klinikum Karlsruhe, Karlsruhe, Germany
- 2Ophthalmology Unit, Department of Experimental Medicine and Surgery, University of Rome Tor Vergata, Italy
Received: April 25, 2018; Published: April 27, 2018
Corresponding author:Emiliano Di Carlo, Department of Ophthalmology, Staedtisches Klinikum Karlsruhe, Moltkestrasse 90, 76133 Karlsruhe, Germany
DOI: 10.32474/TOOAJ.2018.01.000111
Abstract
Pachychoroid neovasculopathy is a relatively new retinal disease, characterized by the presence of Type 1 choroidal neovascularization associated with signs of increased choroidal thickening and hyperpermeability. The latter features are distinctive of pachychoroid spectrum disease, which also includes pachychoroid pigment epitheliopathy, central serous chorioretinopathy and polypoidal choroidal vasculopathy. These pathologies share common features, such as choroidal vascular disfunction, abnormalities of the retinal pigment epithelium without showing typical characteristics of age related macular degeneration and, occasionally, choroidal new vessels. Many recent works have tried to analyze the main aspects of pachychoroid neovasculopathy with the aim to better understand the natural course and the proper management of the disease. Multimodal imaging modality, with the recent advent of the optical coherence tomography angiography, plays a fundamental role to help the physicians to distinguish choroidal neovascularization in pachychoroid disease from neovascular age related macular degeneration. In this review, we summarize the latest updates in the pathogenesis, clinical features, and advances in imaging multimodalities in order to delineate a clearer description of the pachychoroid neovasculopathy. Finally, we propose recommendation guidelines for the diagnosis and management of this relatively new clinical entity. Nevertheless, larger studies and clinical trials are needed to achieve standardized diagnostic criteria and treatment modalities.
Keywords: Pachychoroid neovasculopathy; Pachychoroid disease; Neovascular age related macular degeneration; Neovascularization; Anti-VEGF
Abbreviation: EDI: Enhanced Depth Imaging; SSOCT: Swept Source Optical Coherence Tomography; CSC: Central Serous Chorioretinopathy; ICGA: Indo Cyanine Green Angiography; PEDs: pigment epithelial detachments; PPE: Pachychoroid Pigment Epitheliopathy; CNV: Choroidal Neo Vascularization; PCV: Polypoidal Choroidal Vasculopathy; PVN: Pachychoroid Neovasculopathy; BM: Bruch’s Membrane; OCTA: Optical Coherence Tomography Angiography; FAF: Fundus Auto Fluorescence; Anti-VEGF: Anti- Vascular Endothelial Growth Factors.
Introduction
Definition
The recent great progress in the multimodal imaging of the retina has provided new comprehensions into the understanding of various chorioretinal diseases. Mostly, the development of new OCT softwares, such as the enhanced depth imaging (EDI) [1] and the swept source optical coherence tomography (SS-OCT) [2], has contributed to the significant improvement in the analysis of the morphology and physiology of the choroid. Warrow et al. [3], firstly in 2013, observed the presence of EPR abnormalities in the controlateral eye of patients affected by unilateral acute central serous chorioretinopathy (CSC), including increased choroidal thickness and dilated choroidal vessels on EDI OCT, and choroidal hyperpermeability on indocyanine green angiography (ICGA). The presence of the previous findings with an overlying area of EPR abnormalities, such as drusenoid RPE changes and or small pigment epithelial detachments (PEDs), was considered by the same author “a forme frust” of CSC, giving it the denomination of pachychoroid pigment epitheliopathy (PPE). In the last years, the presence of choroidal neovascularization (CNV) in chronic CSC was well documented [4]. Moreover, an association between CSC and polypoidal choroidal vasculopathy (PCV) has been demonstrated [5]. The findings of the important role played by the thick choroid in PCV has driven the researchers to believe that PCV falls within the pachychoroid spectrum instead of AMD [6]. Therefore, recognizing and precisely diagnosing PPE could play a crucial role to distinguish the possible etiology in those cases, in which the origin of the neovascularization is not clear.
Pang et al. [7] termed as “pachychoroid neovasculopathy” (PVN) the presence of Type 1 neovascularization that occurs overlying an area of focal choroidal thickening, in order to differentiate the “pachychoroid” origin of the pathophysiological mechanism from the other sources of Type 1 neovascularization. PVN represents a new clinical entity of CNV, characterized by thick choroid, RPE alterations and choroidal hyperpermeability. It has been accepted to include it within the spectrum of the pachychoroid disease, together with PPE, CSC and PCV. PVN shows the typical characteristics of Type 1 neovascularization, without the presence of AMD features or degenerative changes. Therefore, the most relevant aspect is the possibility that PVN could masquerade as neovascular AMD, leading to misdiagnosis [8]. The aim of this review is to summarize the main aspects of pachychoroid neovasculopathy within the whole spectrum of pachychoroid disease, from the pathogenetic mechanisms to the management and treatment options.
Pathogenesis
Choroid is a tissue characterized by vascularized and pigmented cells. Five different layers compose the choroid, from the innermost, represented by the Bruch’s membrane (BM), to the outermost, namely suprachoroid lamina. The tree central layers carry out the main functions regarding the pathophysiology of the pachychoroid disease. The choriocapillaris is located right after the BM and consists of the fenestrated veins that supply the outer layer of the retina. In the middle is positioned the Sattler’s layer, formed small oval vascular patterns. Haller’s layer is composed of large outer vascular patterns [9].
The single entities included in the spectrum of pachychoroid disease (PPE, CSC, PVN and PCV) share 3 main common features:
a) Increasing in choroidal thickness.
b) The presence of pathological venous dilation at the level of the Haller’s layer.
c) Thinned choroid in Sattler’s and choriocapillaris layers [10].
Starting from these alterations in the morphology and physiology of the choroid, it seems to exist like a “common thread” in the evolution of the pachychoroid disease, leading to the development of a Type 1 neovascularization and, ultimately, to the formation of polyps. It has been demonstrated that PPE could be caused by the mechanical compression of the choriocapillaris and Sattler’s layer by the dilation of the underlying large outer choroidal vessels located in the Haller’s layer [11]. Choroidal hyperpermeability and congestion play a fundamental role in the pathogenesis of CSC, generating the presence of subretinal fluid [12]. The exact mechanism of CNV formation within PNV is still not well understood, but it could be important to analyze it as a dynamic process in which the growth of new vessels is strictly linked with choroidal flow changements. In the recent literature, was established the association between long-standing CSC and Type 1 CNV [13]. Chronic RPE changes and long standing PED allow the ingrowth of sub RPE endothelial cells [14]. Because PPE and CSC share similar pathophysiological mechanisms, it could be possible that eyes with long standing PPE have a predisposition to develop Type 1 CNV, without the typical manifestations of AMD, such as drusen or degenerative changes [7].
Moreover, Miyake et al. [8] have found that patients affected by PVN have a different genetic susceptibility to AMD compared with patients affected by neovascular AMD, suggesting a different etiology of the two conditions. The recent introduction of the Optical Coherence Tomography Angiography (OCTA) has allowed an additional step forwards to better understand the formation of new vessels within the PVN. Azar et al. [15] have proposed that the ingrowth of new vessels may represent an effort to reform the choriocapillaris in response to the dilation of the outer choroidal layer. This could, in part, explains why the new vessels are localized adjacent to the border of the dilated choroidal vessels.
Diagnosis
Although there are no recommended diagnostic criteria for PVN, there are numerous reports describing the principle features of the single entities within the spectrum of pachychoroid disease [16]. Because PVN represents a neovascular late complication of long standing PPE and chronic CSC [7,17], understanding the clinical characteristics of these 2 pathologies allows to the physicians to make a proper diagnosis. PPE is characterized by the absence of normal tessellation by fundus examination; the presence of thick choroid and large choroidal vessels underlying an area with small elevations of the RPE and, occasionally, small PED, observed by OCT; ICGA findings of choroidal hyperpermeability corresponding to the RPE abnormality areas; hyperautofluorescence recognized with the use of the fundus autofluorescence (FAF). Pang [7] described as “PVN” the findings of Type 1 neovascularization in association with choroidal thickening and dilated choroidal vessels, in the absence of AMD or other degenerative changes, which could eventually progress to PCV. Miyake et al. [8] diagnosed PVN when all of the following criteria were met:
a) CNV in either eye.
b) Subfoveal choroidal thickness > 200 μm in both eyes.
c) No drusen or only non extensive (total area, ≤ 125 μm circle) hard drusen (≤ 63 μm) in both eyes (AREDS category) 1, no AMD.
d) Characteristics of PPE or CSC.
Therefore, it can be affirmed that PVN can be differentiated from neovascular AMD by the absence of soft drusen, the presence of a thick choroid with pachyvessels and younger age at onset of neovascularization.
In the last years the introduction of OCTA has enabled the comprehension of the pachyvessels, mostly regarding the finding of neovascularization below a PED [18,19,15]. Pigment epithelial detachments in eyes affected by pachychoroid disease often shows a shallow, flat and irregular morphology, that closely resembles the “double layer sign”, firstly described by Sato in patients with PCV [20]. Dansignani et al. [18] showed, with the aim of OCTA, that 95% of the shallow irregular PEDs harbored Type 1 CNV, in the form of tangled vascular networks. The previous OCTA findings were confirmed by Azar et al. [15], which described the presence of tangled filamentous new vessels overlying a focal area of thickened choroid in patients with typical characteristics of PPE. Further reports regarding the fundamental role of OCTA in detecting CNV within the pachychoroid spectrum were given by the study of Demirel et al. [19]. Certainly, they demonstrated a greater sensitivity of OCTA, compared to conventional dye angiography (FA and ICGA), in detecting the presence of new vessels in case of shallow and irregular flat PED within pachychoroid disease. It can be assert that the presence of shallow, flat and irregular PED detected by EDI OCT and the OCTA sign of tangled filamentous new vessels in a patient with pachychoroid features, could lead to the diagnosis of pachychoroid neovasculapathy.
Management
Neovascular AMD has been managed with various treatment modalities, comprising laser photocoagulation, surgical removal of fibrovascular tissues, photodynamic therapy (PDT), and intravitreal injections of anti-vascular endothelial growth factors (anti-VEGF). Anti-VEGF injections are currently the standard therapy for neovascular AMD in accordance to the two prospective investigations (the Marina [21] and Anchor [22] studies), demonstrating the improvement and the stabilization of visual acuity. Ranibizumab and Aflibercept have been given regulatory approval for ophthalmological applications, while bevacizumab is used only off-label. The effects of all anti-VEGF agents effect via the inhibition of VEGF-A, while only Aflibercept inhibits also VEGF-B and placental growth factor [23]. Various treatment modalities are available: monthly injections [21,22]; pro re natal treatment [24] and treat and extend modality [25]. Although the management of neovascular AMD and pachychoroid neovasculopathy remains the same, requiring anti-VEGF agents as first line therapy, the genotypical and phenotypical difference between these 2 entities may influence the natural course and the prognosis of the disease. Miyake et al. [8] demonstrated that patients with PVN has a significantly longer retreatment period than those with neovascular AMD after a loading dose of anti-VEGF, in this case Ranibizumab. Hata et al. [26] analyzed the concentration of VEGF into the acqueous humor reporting a significant lower concentration of VEGF in patients affected by PVN in comparison to VEGF concentration in eyes with neovascular AMD. They also found a significant correlation between VEGF concentration and the response to 3 monthly anti-VEGF loading dose treatment, suggesting that high VEGF concentrations at baseline may predict a poor response to anti-VEGF therapy in PVN.
Matsumoto et al. [27] have investigated the efficacy of intravitreal aflibercept treatment using treat and extend regimen in PVN and Type 1 neovascular AMD. They found that treat and extend regimen of intravitreal aflibercept injection may be equally effective in terms of BCVA improvement and exudative changes both in PVN and neovascular AMD. At the same time, it has been observed that: central choroidal thickness decreasing was greater in PVN group; eyes affected by PVN required fewer injections, especially for eyes with signs of polypoidal lesion compared to PVN eyes without polyps. Moreover, Padròn Perez et al. [28] have examined changes in choroidal thickness in patients with PVN treated with intravitreal injections of anti-VEGF. A significant choroidal thickness reduction was detected by the authors after 12 months of anti-VEGF treatment. Furthermore they observed a strict correlation between the number of injections and choroidal thickness, suggesting that more intravitreal injections may lead to a greater choroidal thickness reduction.
Conclusion
Pachychoroid disease spectrum includes 4 different retinal pathologies, once considered as separated single entities, such as PPE, CSC, PVN and PCV. They share common pathogenetic and morphological characteristics, arising from the alteration of choroidal vascular physiology.
Pachychoroid neovasculopathy consists of the following main features:
a) younger age of presentation compared to neovascular AMD.
b) absence of normal fundus tessellation and degenerative changes of AMD, like soft drusen.
c) EDI OCT signs of increased choroidal thickness and veins dilation just below an area of RPE abnormalities.
d) choroidal hyperpermeability detected by ICGA, corresponding to the RPE alterations.
e) shallow irregular PED overlying the area with increased choroidal thickness and dilation of choroidal vein.
f) OCTA finding of tangled filamentous vascular networks.
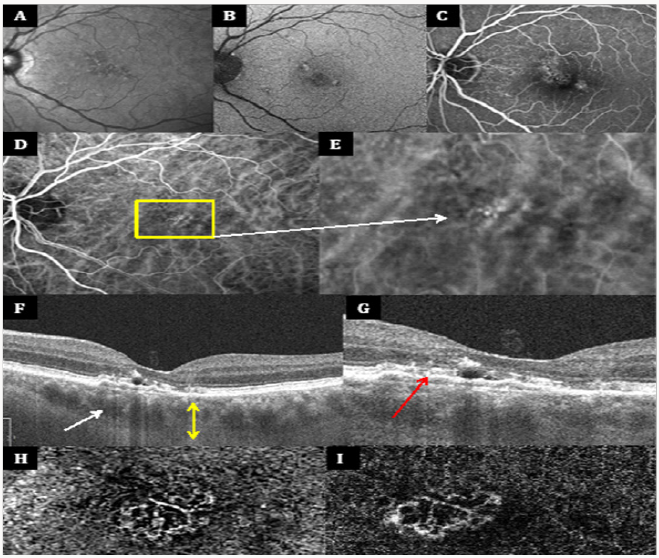
Figure 1 illustrates typical multimodal imaging of a patient with PVN. Anti-VEGF intravitreal injections represent the standard therapy for cases of PVN characterized by Type 1 neovascularization. Patients with PVN required fewer injections in comparison to those with neovascular AMD. Therefore, a proper diagnosis is crucial to establish the appropriate management.
Figure 1(A-I): Multimodal imaging features of pachychoroid neovasculopathy Infrared image (IR), fundus auto fluorescence (FAF) and fluoresce in angiography (FA) shows the presence of few small hard drusen, highlighting the absence of typical degenerative changes of AMD (A-C). Choroidal hyper permeability is demonstrated by indocyanine green angiography (ICGA) images (D), which also shows an hyper fluorescent area corresponding to the vascular network (E) . First enhanced depth imaging optical coherence tomography (EDI OCT) reveals increasing choroidal thickness (yellow arrow with 2 arrowheads) and venous dilations (white arrow) (F), while the second EDI OCT (G) points out the presence of a shallow irregular PED (red arrow) overlying choroidal vascular alterations. Type 1 neovascularization with the features of tangled filamentous new vessels is demonstrated by two different OCT angiography (OCTA) devices (H-I).
References
- Spaide RF, Koizumi H, Pozzoni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146(4): 496-500.
- Potsaid B, Baumann B, Huang D, Barry S, Cable AE, et al. (2010) Ultrahigh speed 1050nm swept source/Fourier domain OCT retinal and anterior segment imaging at 100,000 to 400,000 axial scans per second. Opt Express 18(19): 20029-20048.
- Warrow DJ, Hoang QV, Freund KB (2013) Pachychoroid pigment epitheliopathy. Retina 33(8): 1659-72.
- Fung AT, Yannuzzi LA, Freund KB (2012) Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 32(9): 1829-1837.
- Ahuja RM, Downes SM, Stanga PE, Koh AH, Vingerling JR, et al. (2001) Polypoidal choroidal vasculopathy and central serous chorioretinopathy. Ophthalmology 108(6): 1009-1010.
- Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen SJ, Chen Y, et al. (2018) Polypoidal Choroidal Vasculopathy: Definition, Pathogenesis, Diagnosis, and Management. Ophthalmology 125(5): 708-724.
- Pang CE, Freund KB (2015) Pachychoroid neovasculopathy. Retina 35(1): 1-9.
- Miyake M, Ooto S, Yamashiro K, Takahashi A, Yoshikawa M, et al. (2015) Pachychoroid neovasculopathy and age related macular degeneration. Sci Rep 5: 16204.
- Staurenghi G, Sadda S, Chakravarthy U, Spaide RF (2014) International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel.Proposed lexicon for anatomic landmarks in normal posterior segment spectral domain optical coherence tomography: the IN•OCT consensus. Ophthalmology 121(8): 1572-8.
- Akkaya S (2017) Spectrum of pachychoroid diseases. Int Ophthalmol.
- Lehmann M, Bousquet E, Beydoun T, Behar Cohen F (2015) PACHYCHOROID: an inherited condition? Retina 35 (1): 10-6.
- Tittl M, Polska E, Kircher K, Kruger A, Maar N, et al. (2003) Topical fundus pulsation measurement in patients with active central serous chorioretinopathy. Arch Ophthalmol 121(7): 975-8.
- Freund KB, Zweifel SA, Engelbert M (2010) Do we need a new classification for choroidal neovascularization in age related macular degeneration? Retina 30(9): 1333-1349.
- Zayit Soudry S, Moroz I, Loewenstein A (2007) Retinal pigment epithelial detachment. Surv Ophthalmol 52(3): 227-43.
- Azar G, Wolff B, Mauget Faÿsse M, Rispoli M, Savastano MC, et al. (2017) Pachychoroid neovasculopathy: aspect on optical coherence tomography angiography. Acta Ophthalmol 95(4): 421-427.
- Gallego Pinazo R, Dolz Marco R, Gómez Ulla F, Mrejen S, Freund KB (2014) Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol 3(4): 111-115.
- Quaranta El Maftouhi M, El Maftouhi A, Eandi CM (2015) Chronic central serous chorioretinopathy imaged by optical coherence tomographic angiography. Am J Ophthalmol 160(3): 581-587.
- Dansingani KK, Balaratnasingam C, Klufas MA, Sarraf D, Freund KB (2015) Optical Coherence Tomography Angiography of Shallow Irregular Pigment Epithelial Detachments In Pachychoroid Spectrum Disease. Am J Ophthalmol 160(6): 1243-1254.
- Demirel S, Yanık Ö, Nalcı H, Batıoğlu F, Özmert E (2017) The use of optical coherence tomography angiography in pachychoroid spectrum diseases: a concurrent comparison with dye angiography. Graefes Arch Clin Exp Ophthalmol 255(12): 2317-2324.
- Sato T, Kishi S, Watanabe G, Matsumoto H, Mukai R (2007) Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina 27(5): 589-94.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, et al. (2006) Ranibizumab for neovascular age related macular degeneration. N Engl J Med 355(14): 1419-31.
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, et al. (2006) Ranibizumab versus verteporfin for neovascular age related macular degeneration. N Engl J Med 355(14): 1432-44.
- Stewart MW (2012) Aflibercept (VEGF Trap-eye): the newest anti VEGF drug. Br J Ophthalmol 96(9): 1157-1158.
- Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, et al. (2009) A variable dosing regimen with intravitreal ranibizumab for neovascular age related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 148 (1): 43-58.
- Rayess N, Houston SK 3rd, Gupta OP, Ho AC, Regillo CD (2015) Treatment outcomes after 3 years in neovascular age related macular degeneration using a treat and extend regimen. Am J Ophthalmol 159(1): 3-8.
- Hata M, Yamashiro K, Ooto S, Oishi A, Tamura H, et al. (2017) Intraocular Vascular Endothelial Growth Factor Levels in Pachychoroid Neovasculopathy and Neovascular Age Related Macular Degeneration. Invest Ophthalmol Vis Sci 58(1): 292-298.
- Matsumoto H, Hiroe T, Morimoto M, Mimura K, Ito A, et al. (2018) Efficacy of treat and extend regimen with aflibercept for pachychoroid neovasculopathy and Type 1 neovascular age related macular degeneration. Jpn J Ophthalmol 62(2): 144-150.
- Padrón Pérez N, Arias L, Rubio M, Lorenzo D, García Bru P, et al. (2018) Changes in Choroidal Thickness After Intravitreal Injection of Anti Vascular Endothelial Growth Factor in Pachychoroid Neovasculopathy. Invest Ophthalmol Vis Sci 59(2): 1119-1124.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...