Lupine Publishers Group
Lupine Publishers
Review Article(ISSN: 2644-1209) 
Guide Lines for Wet AMD Treatment Volume 1 - Issue 5
Ahmed Darwish*
- Department of Ophthalmology, Ain Shams University, Egypt
Received: August 28, 2018; Published: September 10, 2018
*Corresponding author: Ahmed Darwish, Professor of ophthalmology, Department of Ophthalmology, Ain Shams University, Vitreoretinal surgery consultant at Dhahran Eye Specialist Hospital, KSA, Egypt
DOI: 10.32474/TOOAJ.2018.01.000121
Abstract
Purpose: To give practical guide lines for the management of neovascular AMD
Methods: To evaluate the above-mentioned item based on recently published data.
Results: Although many therapeutic modalities have been employed for neovascular AMD treatment, yet anti-VEGFs are still the best line of treatment.
Conclusion: Although anti-VEGFs are up till now the best line of treatment for nAMD yet a knowledge of how to use them with or without other modalities is very important to get the best therapeutic results.
Keywords: nAMD; Anti-VEGFs
Introduction
Many therapeutic modalities have been employed for neovascular AMD which includes:
I. Macular laser photocoagulation [Macular Photocoagulation Study (MPS) can result in preventing severe loss of vision in about 50 percent of treated patients with extra/ juxta foveal CNV, with about 50 percent of patients developing recurrent choroidal neovascular membrane (CNVM).
II. Photodynamic therapy (PDT) with verteporfin acts via activating a photosensitizing dye within the pathologic vessels by infrared laser leading to occlusion of choroidal new vessels with minimal damage to the retina. PDT can prevent 3-line vision loss in about 49 to 77 percent of treated patients but seldom improves vision [1].
III. With the establishment of VEGF as the main cause for the development and progression of neovascularization, novel agents to block them and thereby preventing further progression was sought for. With the advent of anti-VEGF agents, the treatment for neovascular AMD has completely changed, with dramatic outcomes.
Anti-VEGFs
The inclusion of ranibizumab, a nonspecific VEGF inhibitor further refined the results of anti-VEGF therapy for neovascular AMD [2]. The MARINA study evaluated the effect of ranibizumab injection in patients with minimally classic or occult CNV. The conclusion of Marina study was that monthly IVL injection for 2 years prevented vision loss and improved mean VA in patients with minimally classic / occult CNV secondary to AMD. The conclusion of the ANCHOR trial was that Lucentis was superior to PDT as treatment of predominantly classic NAMD [3- 9]. The Comparison of AMD Treatments Trial Study (CATT study) trial was primarily designed to determine if bevacizumab works as well as ranibizumab in terms of visual outcomes (a difference of <5 letters), and also to identify any safety differences between the two drugs.
Visual outcome results:
When comparing ranibizumab monthly to bevacizumab monthly, the CATT study demonstrated no difference between the two drugs, with patients in both groups gaining more than 8 letters on the eye chart on average over the course of a year and the results were maintained over 2 years.
Safety Outcomes
The rate of ocular infection following injection of medication was similar with the two drugs [10].
A similar head to head comparison trial between the two drugs was the alternative treatments to Inhibit VEGF in Age-related choroidal Neovascularization [IVAN]: 1year results concluded similar efficacy of both drugs [11].
Treatment Protocols: Primarily Designed for AMD
Ranibizumab: As-needed Regimen The Prospective OCT Imaging of Patients with Neovascular AMD Treated with Intraocular Ranibizumab [PrONTO] Study: In this study patients received 3 consecutive monthly injections of 0.5 mg ranibizumab and were then followed monthly and retreated if there was an increase in OCT central retinal thickness [CRT] of at least 100 microns or a loss of best-corrected ETDRS VA of 5 letters or more. In the PrONTO study, VA outcomes were comparable with those reported in ranibizumab phase III clinical studies, but with fewer intravitreal injections [12]. The Sailor [13], Sustain [14] and Horizon [15] trials are other as needed regime studies. Overall, these studies support frequent follow-up and individualized retreatment to achieve the best visual acuity gains with the as-needed treatment regimen.
Ranibizumab: Treat-and-Extend Regimen:
Treat-and-extend dosing regimen involves increasing intervals between treatment up to 10 weeks as long as no fluid is present on OCT. If fluid is present, the interval between treatments is shortened. Oubraham found that at one-year, mean gain in VA was greater in the treat and-extend group than in the as-needed group [+10.8 versus+2.3 letters, resp.]. Eyes in the treat-and-extend group received significantly more mean injections [7.8 versus 5.2] [16]. Similar trials were also done with Bevcizumab with similar results [17-25].
Aflibercept as Compared to Other Anti-VEGFs
A. Aflibercept is a soluble decoy receptor produced by fusing all-human DNA sequences of the second immunoglobulin domain of human VEGFR1 and the third immunoglobulin domain of human VEGFR2, which then fused to the Fc region of human IgG1.2. The intravitreal half-life of aflibercept is 4.7 days in rabbit eyes, which is longer than ranibizumab [2.9 days] and comparable with bevacizumab [4.3 days].
B. The combined high affinity and longer half-life has led to a calculated duration of effect of a single intravitreal injection of 2 mg aflibercept of 48-83 days. Monthly treatment with aflibercept has been shown to improve the vision in exudative AMD in 2 clinical trials. VIEW [VEGF Trap-Eye: Investigation of Efficacy and Safety in wet AMD] 1 and View 2 showed that, at 1 year, aflibercept treatment [0.5, 2 mg monthly, or 2 mg every 2 months after three initial monthly doses] was non-inferior and clinically equivalent to ranibizumab [0.5 mg] given monthly [26].
C. Aflibercept therapy appears to be beneficial in a subset of patients with neovascular age-related macular degeneration who exhibit recurrent or resistant intra-retinal or subretinal fluid following multiple injections with either bevacizumab or ranibizumab [27].
Anatomical Measures as Predictors of Visual Outcomes in Ranibizumab-Treated Eyes with Neovascular Age- Related Macular Degeneration:
a) First and foremost, an initial anatomical [according to FFA and/or OCT analyses] or visual improvement after three monthly ranibizumab injections does not guarantee longterm success. For eyes with FFA lesion activity at Month 3, CFT>/=200mm at Month 3, and qualitative OCT activity at Months 2 and Month 3 the average BCVA gain from 3 monthly loading doses of ranibizumab was lost after switching to quarterly dosing [every 3 months], and eyes lost vision compared with baseline at Months 12 and 24.
b) Second, it appears that the longer anatomical improvements were maintained [according to FFA or OCT], the more likely it was that the BCVA benefits of ranibizumab persisted on a quarterly dosing regimen. Eyes with inactive FFA lesions at Month 5 or inactive OCT lesions at Month 5 or Month 8 were much more likely to maintain their BCVA gains.
c) While a surprisingly low number of eyes demonstrated inactive FFA lesions after 3 loading doses of ranibizumab (i.e., 10% at Month 3), eyes with a dry FFA showed the strongest association with BCVA outcomes at Months 12 and 24. At the same 3-month time point, 60% of evaluated eyes were dry on qualitative OCT grading. This disparity may result from the sampling error introduced by having only two scans available for grading (rather than all 6 radial line scans available from a Stratus macular thickness map or the greatly increased sample size of currently available spectral-domain OCT devices). It is also known that an effective RPE pump sometimes keeps the retina dry and gives a “dry” OCT reading, despite active CNV leakage [28].
Comparison of Spectral-Domain and Time-Domain Optical Coherence Tomography in the Detection of Neovascular Age- Related Macular Degeneration Activity:
a. With high-resolution volumetric SD-OCT imaging, physicians are capable of detecting signs of exudative AMD activity more precisely. Time domain platforms are less likely to identify active exudative disease activity; this could potentially lead to undertreatment of active neovascular AMD.
b. Both volumetric and raster scans collect data in the same way, via parallel B-scans. The important difference being that volumetric scanning includes more parallel B-scans, in a denser array, providing higher resolution and the ability to render a three-dimensional image. For example, with the Cirrus platform, the 5-line raster algorithm uses only 5 B-scans compared with 128 B-scans used with the volumetric scan [29-30] (Figure 1).
c. Some areas of exudative activity that oriented more vertically were better visualized with radially oriented SD imaging compared with the more traditional horizontal raster scanning patterns [30] (Figure 2).
Figure 1: Examples where the raster scan did not image the retinal area with exudative activity because of sampling error. Spectralis volume scan (top) versus raster (bottom) [29].
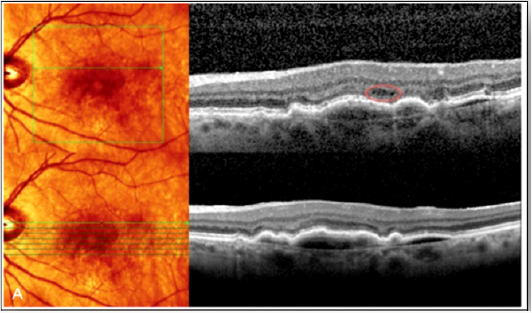
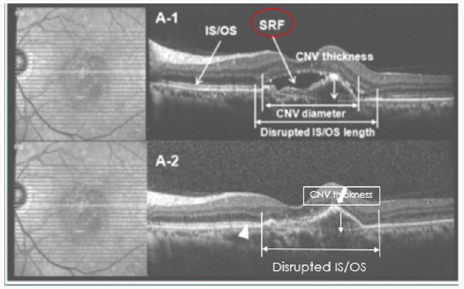
Figure 2: Correlation of Foveal Microstructural Changes with Vision after Anti–Vascular Endothelial Growth Factor Therapy in Age-Related Macular Degeneration.
If after 3 loading doses of Ranibizumab the CNV activity disappeared but the visual acuity did not improve as expected, this might be either due to a disrupted IS/OS line or a thick CNV membrane. In conclusion Visual acuity was most improved when the disrupted IS/OS line was better restored, and CNV thickness was more decreased [31] (Figure 3).
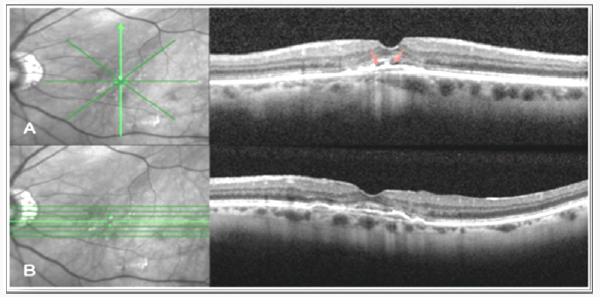
Correlation of Spectral Domain Optical Coherence Tomography Characteristics with Visual Acuity in Eyes with Sub-foveal Scarring After Treatment for Wet Age-Related Macular Degeneration
In a case series, visual acuity in cases of sub foveal scarring was affected mainly by the integrity of the IS/OS and external limiting membrane lines [32] (Figure 4).
Figure 4: Correlation of Spectral Domain Optical Coherence Tomography Characteristics with Visual Acuity in Eyes with Sub foveal Scarring after Treatment for Wet Age-Related Macular Degeneration.
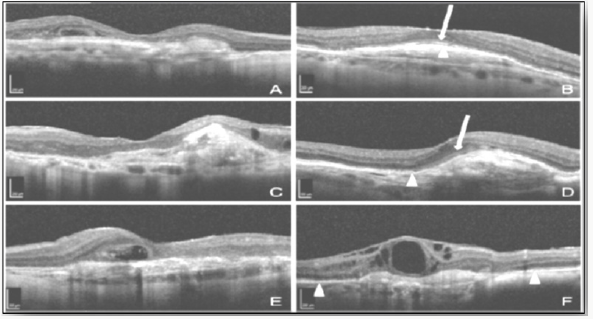
Spectral domain OCT scans of patients with sub foveal scarring:
A. Thin fovea with subfoveal scarring, VA: Counting fingers.
B. Intact IS/OS junction (arrowhead) and ELM (arrow) in fovea, VA: 20/32.
C. Normal foveal thickness with no IS/OS or ELM seen, VA: Counting fingers.
D. Intact ELM at fovea, with intact IS/OS near fovea, VA: 20/40.
E. Foveal cystoid degeneration, VA: 20/800.
F. Cystoid degeneration with disrupted IS/OS within central 1,000 mm, but intact near fovea, VA: 20/80 [32].
Response 0f Pigment Epithelial Detachments to Intravitreal Aflibercept among Patients with Treatment-Resistant Neovascular Age-Related Macular Degeneration
Figure 5: Three PED types were identified on OCTs; hollow, solid and mixedss. The hollow type showed the best response to aflibercept treatment while the solid type was the worst in response.
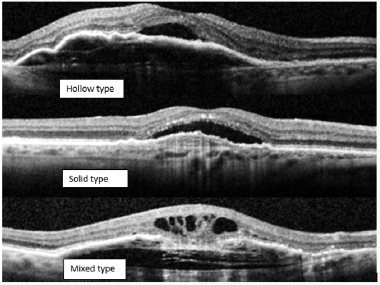
Three PED types were identified on OCT; hollow, solid and mixed. The hollow type showed the best response to aflibercept treatment while the solid type was the worst in response (Figure 5).
a) Hollow: are hypo reflective and contain fluid exudate
b) Solid: hyper reflective and represents fibrinous leakage or fibrovascular proliferation, suggesting active neovascularization.
c) Mixed:
i) Vision loss associated with PEDs seems to be largely nonreversible , even with structural reduction of the lesion.
ii) Retinal pigment epithelium tears may complicate treatment of PEDs during treatment with intravitreal anti-VEGF therapy. Larger vascularized PEDs that have a higher intraluminal pressure are at a significantly greater risk of producing RPE tears after anti- VEGF therapy (especially in the early stages of ttt), with acute vision loss [33].
Unfavorable Anatomical Response to Anti VEGFs
I. Some patients, however, have a good initial response to Avastin & Lucentis with resolution of fluid, but then later become resistant to further treatment and develop recurrent exudation with vision loss. The mechanism of this resistance to treatment with these drugs is not known, but one possibility is tolerance or tachyphylaxis, manifested by a decreased response over time to repeated treatment with a medication. Tachyphylaxis sometimes can be reversed by increasing the dose or halting therapy for a period of time before reinstating the same treatment.
II. Aflibercept therapy as mentioned above, appears to be beneficial in a subset of patients with neovascular age-related macular degeneration who exhibit recurrent or resistant intraretinal or subretinal fluid following multiple injections with either bevacizumab or ranibizumab [27].
Response of Type 3 Neovascularization to Anti-VEGF Treatment
a) The CME and sub-RPE fluid associated with Type 3 neovascularization resolve briskly with intravitreal anti-VEGF therapy, typically after only one or two injections.
b) A recent, longitudinal prospective study examining the response of Type 3 lesions to anti-VEGF therapy demonstrated that all eyes had stable or improved vision at 3 years of follow-up after a mean of 9.4 injections during that time. The visual prognosis was excellent [34].
Combination Therapies for Wet AMD
Role of Additional Dexamethasone for the Management of Persistent or Recurrent Neovascular Age Related Macular Degeneration Under Ranibizumab Treatment
a) The efficacy of a combination therapy of intravitreal ranibizumab together with a dexamethasone implant in comparison with ranibizumab monotherapy in persistent or recurrent neovascular age-related macular degeneration was studied and it was found that combined therapy delays retreatment in patients with persistent/recurrent neovascular age-related macular degeneration and an overall reduction in required ranibizumab retreatments compared with ranibizumab monotherapy with consistent functional outcomes. [35].
b) The expectations on the improved effect of a combination therapy lie on the multifactorial pathogenesis of nAMD involving angiogenesis and inflammation. As CNV persist under monotherapy, a combined approach seems to be reasonable to decelerate disease progression. Corticosteroids act because of their anti-inflammatory, antiangiogenic, and antiedematous effects [36-38]. Hence, additional corticosteroids seem to have the ability to target chronic inflammation when combined with anti-VEGF. In addition, a decrease in effect during an anti- VEGF monotherapy has been reported, and desensitization of tachyphylaxis by adding corticosteroids in chronic CNV was suggested [39].
Anti- VEGF Combined with Photodynamic Therapy
The combination has an additive or synergistic effect; PDT targets the vascular component Anti-VEGF targets the mediators of the angiogenic cascade and counteracts up-regulation of angiogenic factors that occur after PDT treatment. The combination causes reduction of re-treatment rate BUT may not achieve equivalent visual acuity outcomes [40-42].
Avastin Triple Therapy
a. The aim of this treatment is to combine Avastin with PDT and Dexamethasone. First PDT Light dose 42j/cm is delivered in 70 sec then after 16h. Intravitreal injection of 800mcg dexamethasone plus 1.5mg Avastin are given.
b. Triple therapy in one study was found to result in a good VA outcome with lower cost compared to repeated injections. Other studies, however, failed to show any benefit of the triple therapy as compared to anti-VEGF monotherapy [43-44].
References
- Bressler NM, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group (2001) Photodynamic therapy of sub foveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol 119(2): 198- 207.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. (2004) Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23): 2335-2342.
- Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M (1998) Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol 341(2-3): 309-315.
- Gordon MS, Margolin K, Talpaz M, Sledge GW Jr, Holmgren E, et al. (2001) Phase I safety and pharmacokinetic study of recombinant human antivascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 19(3): 843-850.
- Ferris F, Fine SL, Hyman L (1984) Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 102(11): 1640-1642.
- Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, et al. (2007) Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 114(2): 246-252.
- Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, et al. (2007) Improved vision-related function after ranibizumab treatment of neovascular agerelated macular degeneration: Results of a randomized clinical trial. Arch Ophthalmol 125(11): 1460-1469.
- Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, et al. (2007) Ranibizumab for predominantly classic neovascular age-related macular degeneration: Subgroup analysis of first- year ANCHOR results. Am J Ophthalmol 144(6): 850-857.
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, et al. (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 116(1): 57-65.
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. (2012) Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ophthalmology 119(7): 1388-1398.
- IVAN Study Investigators, Chakravarthy U, Harding SP, Rogers CA, Downes SM, et al. (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology 119(7): 1399-1411.
- Lalwani A, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, et al. (2009) A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: Year 2 of the PrONTO study. Am J Ophthalmol 148(1): 1-3.
- Boyer S, Heier JS, Brown DM, Francom SF, Ianchulev T, et al. (2009) A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular agerelated macular degeneration. Ophthalmology 116(9): 1731-1739.
- Mitchell P, Korobelnik JF, Lanzetta P, Holz FG, Prunte C, et al. (2010) Ranibizumab (Lucentis) in neovascular age-related macular degeneration: Evidence from clinical trials. Br J Ophthalmol 94(1): 12- 13.
- (2009) Ranibizumab safe and effective over long term in Horizon extension study. Ophthalmology Times.
- Oubraham H, Cohen SY, Samimi S, Marotte D, Bouzaher I, et al. (2010) Inject and extend dosing versus dosing as needed: A comparative retrospective study of ranibizumab in exudative age-related macular degeneration. Retina 31(1): 26-30.
- Tufail A, Patel PJ, Egan C, Hykin P, da Cruz L, et al. (2010) Bevacizumab for neovascular age related macular degeneration (ABC Trial): Multicentre randomised double masked study. BMJ 340(7761): 1398.
- Fong KCS, Kirkpatrick N, Mohamed Q, Johnston RL (2008) Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration using a variable frequency regimen in eyes with no previous treatment. Clin Exp Opthalmol 36(8): 748-755.
- Mekjavic J, Kraut A, Urbancic M, Lenassi E, Hawlina M (2009) Efficacy of 12-month treatment of neovascular age-related macular degeneration with intravitreal bevacizumab based on individually determined injection strategies after three consecutive monthly injections. Acta Ophthalmolo 89(7): 647-653.
- Leydolt C, Michels S, Prager F, Garhoefer G, Georgopoulos M, et al. (2010) Effect of intravitreal bevacizumab (Avastin) in neovascular age-related macular degeneration using a treatment regimen based on optical coherence tomography: 6- and 12-month results. Acta Ophthalmolo 88(5): 594-600.
- Luu ST, Gray T, Warrier SK, Patel I, Muecke JS, et al. (2010) Retrospective study of an as required dosing regimen of intravitreal bevacizumab in neovascular age-related macular degeneration in an Australian population. Clin Exp Ophthalmol 38(7): 659-663.
- Arevalo F, Snchez JG, Wu L, Berrocal MH, Alezzandrini AA, et al. (2010) Intravitreal bevacizumab for subfoveal choroidal neovascularization in age-related macular degeneration at twenty-four months: The panamerican collaborative retina study. Ophthalmol 117(10): 1974-1981.
- Bashshur F, Haddad ZA, Schakal AR, Jaafar RF, Saad A, et al. (2009) Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: The second year of a prospective study. Am J Ophthalmol 148(1): 59-65.
- Gupta P, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, et al. (2010) A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration: Clinical and economic impact. Ophthalmology 117(11): 2134-2140.
- Treat and extend therapy a popular, cost-effective approach to treating neovascular AMD (2011) Ocular Surgery News US Edition, USA.
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, et al. (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119(12): 2537-2548.
- Kumar N, Marsiglia M, Mrejen S, Fung AT, Slakter J, et al. (2013) Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina 33(8): 1605-1612.
- Brown DM, Tumio L, shapiro H, Pier Study Group (2013) Anatomical Measures As Predictors Of Visual Outcomes In Ranibizumab-Treated Eyes With Neovascular Age-Related Macular Degeneration. Retina 33(1): 23-34.
- Major JC, Wykoff CC, Mariani AF, Chen E, Croft DE, et al. (2014) Comparison of Spectral-Domain and Time-Domain Optical Coherence Tomography in the Detection of Neovascular Age-Related Macular Degeneration Activity. Retina 34(1): 48-54.
- Sayanagi K, Sharma S, Yamamoto T, Kaiser PK (2009) Comparison of spectral-domain versus time-domain optical coherence tomography in management of age-related macular degeneration with ranibizumab. Ophthalmology 116(5): 947-955.
- Shin HJ, Chung H, Kim HC (2013) Correlation of Foveal Microstructural Changes with Vision After Anti–Vascular Endothelial Growth Factor Therapy in Age-Related Macular Degeneration. Retina 33(5): 964-970
- Emam S, Chhablani J, Barteselli G, Wang H, Lee SN, et al. (2013) Correlation of Spectral Domain Optical Coherence Tomography Characteristics with Visual Acuity in Eyes with Subfoveal Scarring after Treatment for Wet Age Related Macular Degeneration. Retina 33(6): 1249-1257.
- Broadhead GK, Hong T, Zhu M, Li H, Schlub TE, et al. (2015) Response of Pigment Epithelial Detachments to Intravitreal Aflibercept Among Patients with Treatment-Resistant Neovascular Age-Related Macular Degeneration. Retina 35(5): 975-981.
- Nagiel A, Sarraf D, Sadda SR, Spaide RF, Jung JJ, et al. (2015) Type 3 Neovascularization Evolution, Association With Pigment Epithelial Detachment, and Treatment Response As Revealed By Spectral Domain Optical Coherence Tomography. Retina 35(4): 638-647.
- Rezar-Dreindl S, Eibenberger K, Buehl W, Georgopoulos M, Weigert G, et al. (2017) Role of Additional Dexamethasone for The Management of Persistent or Recurrent Neovascular Age Related Macular Degeneration Under Ranibizumab Treatment. Retina 37(5): 962-970.
- Jonas JB, Libondi T, Golubkina L, Spandau UH, Schlichtenbrede F, et al. (2010) Combined intravitreal bevacizumab and triamcinolone in exudative age-related macular degeneration. Acta Ophthalmol 88(6): 630-634.
- Tozer K, Roller AB, Chong LP, Sadda S, Folk JC, et al. (2013) Combination therapy for neovascular age-related macular degeneration refractory to anti-vascular endothelial growth factor agents. Ophthalmology 120(10): 2029-2034.
- Veritti D, Sarao V, Lanzetta P (2013) Bevacizumab and triamcinolone acetonide for choroidal neovascularization due to agerelated macular degeneration unresponsive to antivascular endothelial growth factors. J Ocul Pharmacol Ther 29(4): 437-441.
- Schaal S, Kaplan HJ, Tezel TH (2008) Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology 115(12): 2199-2205.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, et al. (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355(14): 1419-1431.
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, et al. (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355(14): 1432-1444.
- Forte R, Bonavolonta P, Benayoun Y, Adenis JP, Robert PY (2011) Intravitreal ranibizumab and bevacizumab in combination with fullfluence verteporfin therapy and dexamethasone for exudative agerelated macular degeneration. Ophthalmic Res 45(3): 129-134.
- Bakri SJ, Couch SM, McCannel CA, Edwards AO (2009) Same-day triple therapy with photodynamic therapy, intravitreal dexamethasone, and bevacizumab in wet age-related macular degeneration. Retina 29(5): 573-578.
- Augustin AJ, Puls S, Offermann I (2007) Triple therapy for choroidal neovascularization due to age-related macular degeneration: Verteporfin PDT, bevacizumab, and dexamethasone. Retina 27(2): 133-140.