Lupine Publishers Group
Lupine Publishers
Research ArticleOpen Access
Effects of Long Term Use of Prostaglandin Analogues with and Without Preservative on Normal Conjunctival Flora of Glaucoma Patients Volume 2 - Issue 5
Rupankar Sarkar1*, Humayoun Ashraf1, Syed Wajahat Ali Rizvi1 and Sameer Ashraf2
- 1Institute of Ophthalmology, Jawaharlal Nehru Medical College, Faculty of Medicine, Aligarh Muslim University (AMU), Aligarh, India
- 2Ratanjyoti netralaya & Apollo hospital, India
Received:April 12, 2020; Published:April 23, 2020
Corresponding author: Rupankar Sarkar, Institute of Ophthalmology, JN Medical College, AMU., Aligarh, India
DOI: 10.32474/TOOAJ.2020.02.000150
Abstract
Purpose: The effects of long term use of Benzalkonium chloride-preserved prostaglandin analogue eye drop on conjunctival bacterial flora was investigated and compared with preservative-free prostaglandin analogue eye drop
Methods: Conjunctival swabs were collected from 48 patients of glaucoma who had instilled either BAK-preserved latanoprost or preservative-free travoprost eye drops for 1 year. The bacterial characteristics and sensitivity patterns of the bacterial isolates from the conjunctival swabs of the two groups were compared between each other and also with conjunctival bacterial flora from normal healthy controls.
Results: There was no significant difference in culture positivity among the three groups (p = 0.99). 100% (13/13) isolates from Latanoprost group were Gram positive organisms. In the Travoprost group, 12 out of 14 isolates (85.7%) from 13 eyes were gram positive while the rest 2 bacteria (14.3%) were gram negative. In Controls, 14 out of 16 (87.5%) isolates were gram positive and the rest were gram negative. Methicillin-resistance was seen in 72.7% (8/11) of the CONS isolated from Latanoprost group, while 45.5% (5/11) of the CONS isolated from preservative free travoprost group were Methicillin resistant. While in the controls 45.4% (5/11) of the isolated CONS were Methicillin resistant. The occurrence of Methicillin resistant CONS (MR CONS) was higher in latanoprost group than the other two groups, though not statistically significant. Resistance to commonly used antibiotics was comparatively higher in BAK-preserved latanoprost group. Not a single gram positive bacteria was resistant to Vancomycin
Conclusion: Long-term use of prostaglandin analogues for glaucoma may adversely affect the antibiotic-resistance of indigenous bacterial flora of the eye by increasing the resistance against common antibiotics including Methicillin resistance.
Introduction
In recent times, it has been suggested that bacteria may acquire resistant genes or gene mutation might take place in bacteria, imparting resistance to antiseptics. This is mediated by the discharge of the antiseptic out of bacterial cells. Since this mechanism is not drug specific, bacteria resistant to antiseptics can also acquire cross-resistance to antimicrobial drugs [1,2]. Such effects can be anticipated for the conjunctival bacterial flora of patients who are on long-term BAK preserved topical antiglaucoma therapy. Ohtani and associates have demonstrated higher frequency of isolation of Methicillin-resistant Staphylococcus epidermidis (MRSE) in patients instilling BAK-preserved latanoprost as compared to patients instilling travoprost without BAK and also lower susceptibility rates of S. epidermidis in the former group for levofloxacin, gatifloxacin, moxifloxacin and tobramycin [3]. Treatment of glaucoma is aimed at lowering of IOP. Medical therapy with topical monotherapy, mostly with prostaglandin analogues (PGA) is the first step in treating glaucoma as per the European Glaucoma Society Guidelines [4]. Many PGAs contain preservatives which are associated with increased ocular side effects, the most common preservative being benzalkonium chloride (BAK). BAK is a quarternary ammonium compound and exerts its antimicrobial effect by disrupting the cell membrane resulting in cell death of the organism. Research regarding the effects of PGAs and BAK on the ocular flora is unfortunately sparse. In our study we have tried to find out the effects of PGAs with and without BAK on the sensitivity and resistance pattern of conjunctival bacterial flora in patients on long term therapy with these medications.
Methods
This was a prospective study approved by the institutional ethics committee of the faculty of medicine, Aligarh Muslim University and was conducted in accordance with the Declaration of Helsinki and ethical guidelines. The analysis was conducted on 48 eyes from 48 patients of glaucoma and 30 eyes from 30 controls. 48 patients of glaucoma and 30 controls were enrolled for the study between December 2017 and July 2018 who had attended ophthalmology OPD of Jawaharlal Nehru Medical College, AMU and glaucoma clinic, Institute of Ophthalmology, AMU. The newly diagnosed patients of glaucoma who were started on prostaglandin analogues were recruited in the study. All subjects had provided informed consent for their participation in the study. Patients who were newly diagnosed to have glaucoma were started on either of the two eye drops:
• Tovaxo (Travoprost 0.004%, preservative-free; Ajanta Pharmaceuticals)
• 9PM (Latanoprost 0.005%, BAK 0.02%; Cipla Pharmaceuticals)
Inclusion criteria for glaucoma patients included increased iop, characteristic glaucomatous cupping, characteristic visual field loss on perimetry, evidence of glaucomatous damage to retinal nerve fiber and/or macular ganglion cell complex on OCT. Exclusion criteria for glaucoma patients were use of eye drops other than prostaglandin analogues (tovaxo or 9pm), history of ocular surgery, topical or systemic administration of antimicrobial agents in the past 2 weeks, suspected bacterial, fungal or viral infection, poorly controlled ocular or systemic disease. Inclusion criterion for controls was no history of using any eye drops or systemic antimicrobial drugs within the last 12 weeks. The patients were followed up from the time of initiation of therapy and at the end of one year of follow up, conjunctival swabs were collected from the eyes of the patients. A fresh sterile swab stick in a sealed sterile container was obtained for every patient. The cotton tip of the swab was moistened with fresh sterile normal saline. The patient was asked to look up and with the lower lid pulled down, the saline moistened sterile cotton tipped swab stick was gently and swiftly swabbed across the exposed lower fornix with rolling motion. The swabs were quickly transported to the microbiology laboratory.
Laboratory Procedure
Direct microscopy of gram stained smears were performed for all the collected specimens to study the presence of gram positive cocci. All the samples were inoculated on 5% sheep blood agar (BA), McConkey Agar, Chocolate agar and Mannitol salt agar. These were incubated at 35 ̊ C for 18-24 hours. The organisms were identified on the basis of morphology, culture characteristics and biochemical tests. The staphylococcal isolates were identified by colony morphology, pigment production, gram staining and biochemical tests like coagulase test, catalase test. Antibiotic Susceptibility was done by Kirby-Bauer’s disc diffusion method. Following antibiotic discs were used For staphylococcus species (Gram positive panel)
Amikacin (30 μg), Cefoxitin (30 μg), Vancomycin (30 μg), Moxifloxacin (5 μg), Cotrimoxazole (25 μg), Clindamycin (2 μg)
For enterobacteriaceae (Gram negative panel)
Amikacin (30 μg), Ceftriaxone (30 μg), Cefexime (30 μg), Ceftriaxone + sulbactam (30/75 μg),
Gentamicin (10 μg), Tigecycline (15 μg), Moxifloxacin (5 μg), Cotrimoxazole (25 μg)
For pseudomonas species
Amikacin (30 μg), Moxifloxacin (5 μg), Ceftazidime (30 μg), Cefepime (30 μg), Gentamicin (10 μg)
Statistical Analysis: The culture positivity rate, distribution of bacterial species and susceptibility to different antibiotics were documented for each PGA treated group and controls and were compared between the groups. One-way analysis of variance (oneway ANOVA) was a technique used to compare means of three or more samples for numerical data (using the F distribution). A chi-squared test (χ2 test) was any statistical hypothesis test wherein the sampling distribution of the test statistic is a chisquared distribution when the null hypothesis is true. Unpaired proportions were compared by Chi-square test or Fischer’s exact test, as appropriate. p-value ≤ 0.05 was considered as statistically significant.
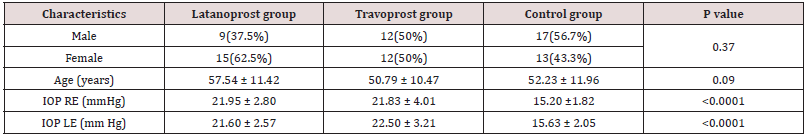
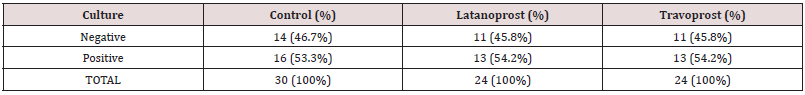
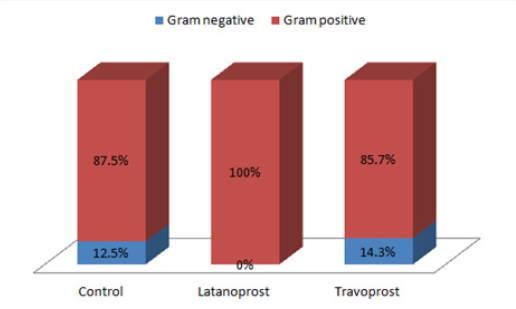
The patients and controls were comparable in terms of age and sex. On performing post hoc test, for both the eyes, the difference in IOP between latanoprost and travoprost was not significant but the difference between controls and the PGA groups were significant. Table 2 shows the rate of culture positivity among the treatment groups and controls. There was no significant difference in culture positivity among the three groups (p = 0.99). Table 2: Rate of culture positivity among Travoprost, Latanoprost and control group Table 3 shows the different bacteria isolated from different groups and their respective numbers and percentages. The Table 4 also depicts the number of eyes in which there was no growth of any organism.
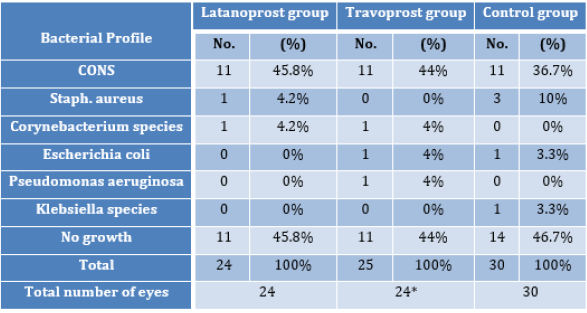
*One eye from travoprost group showed growth of two different organisms; hence despite having 24 eyes in this group the total number of bacteria was 25. Figure 1 shows the distribution of gram positive and gram negative bacteria among the different groups. As shown in Figure 1, 100% (13/13) isolates from Latanoprost group were Gram positive organisms. In the Travoprost group, 12 out of 14 isolates (85.7%) from 13 eyes were gram positive while the rest 2 bacteria (14.3%) were gram negative. In Controls, 14 out of 16 (87.5%) isolates were gram positive and 2 (12.5%) were gram negative. Of all the isolates obtained from all the three groups aggregated, coagulase negative staphylococcus (CONS) accounted for 78.5% (33 out of 42 culture positive eyes) and Staphylococcus aureus accounted for 9.5% (4 out of 42 culture positive eyes). Gram positive bacilli were isolated, which were Corynebacterium species (diphtheroids), accounting for 4.7% of the culture positive cases in our study. Various species of Gram negative bacilli isolated in our study included Escherichia coli accounting for 4.7% (2 out of 42 culture positive eyes), Klebsiella species accounting for 2.3% (1 out of 42) and Pseudomonas aeruginosa accounting for 2.3% (1 out of 42 culture positive eyes). Tables 4, 5 and 6 show the susceptibility patterns of the gram positive organisms in latanoprost group, travoprost group and control group respectively. The only strain of Escherichia coli isolated from one patient of travoprost group was resistant to multiple antibiotics. It was only sensitive to higher antibiotics like Minocycline, Polymixin, Colistin and Tigecycline. The Escherichia coli isolated from one eye of Control group was sensitive to all the regular antibiotics in the gram negative panel of antibiotics. Hence susceptibility to higher antibiotics was not tested further. The Klebsiella species isolated from one eye of Control group was sensitive to all the regular antibiotics in the gram negative panel of antibiotics except Cefexime.
Table 5: Comparison of susceptibility patterns of gram positive bacteria between controls and preservative-free travoprost group.
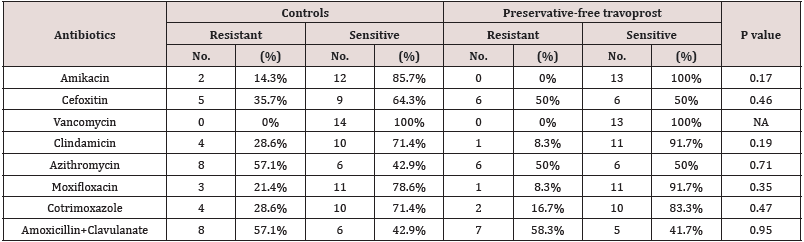
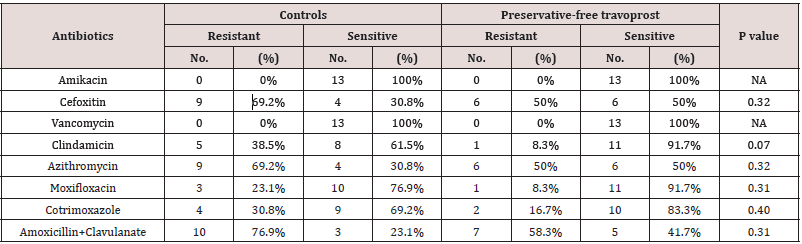
Table 6: Comparison of susceptibility patterns of gram positive bacteria between BAK-preserved latanoprost group and preservativefree travoprost group.
Discussion
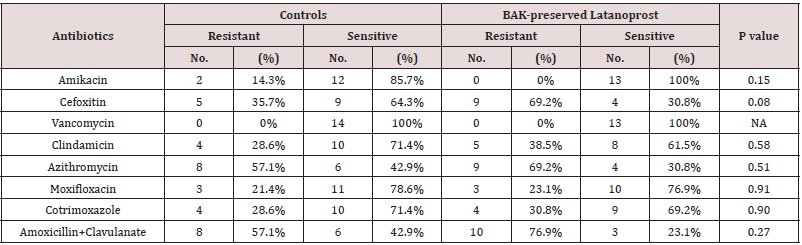
In the current study, 72.7% (8/11) of the CONS isolated from Latanoprost group were Methicillin resistant while 45.5% (5/11) of the CONS isolated from preservative free travoprost group were Methicillin resistant. While in the controls 45.4% (5/11) of the isolated CONS were Methicillin resistant. The occurrence of Methicillin resistant CONS (MR CONS) was higher in latanoprost group than the other two groups, though not statistically significant. similar finding has been reported by Ohtani et al in a study exactly similar to this present study [3]. In the study by Olson et al the percentage of Methicillin resistant staphylococci isolated from the conjunctiva of cataract patients was 38.5% [5], nearly encroaching the figure we found in the control group of our study. The emergence of Methicillin resistance among the conjunctival bacterial flora of glaucoma patients during one year period of use of BAK-preserved latanoprost seemed to be alarming. In this study, the maximum resistance (69.2% of the isolates) to Azithromycin was seen in patients instilling BAK-preserved latanoprost while the patients instilling preservative-free travoprost had 50% of the isolates resistant to Azithromycin and the controls had 57.1% of the isolates resistant to Azithromycin. These figures are more or less at par with the figures obtained from the TRUST and ARMOR studies [6.7]. In the study of Mshangila et al CONS and Staphylococcus aureus had similar resistance to Erythromycin (38.5% and 31%) [8]. In the present study susceptibility to Erythromycin was not tested but to Clindamycin, which is another antibiotic in the same category (Macrolide group of antibiotics) as Erythromycin, was tested. In the control group, 28.6% were found resistant, in preservative-free travoprost group only 8.3% were resistant while in BAK-preserved latanoprost group 38.5% were resistant. The lower rates of resistance in this study is probably because Clindamycin is a higher generation antibiotic than Erythromycin. Interestingly, in the study done by Aghadoost and Khorshidi, >95% of the CONS strains were resistant to Penicillin [9]. In the current study, susceptibility to Penicillin was not recorded, however, the same to Amoxyxillin+Clavulanate was noted. The controls and the preservative-free travoprost group had about 50% of the strains resistant to Amoxycillin+Clavulanate, while the BAK-preserved latanoprost group had 76.9% of the isolates resistant to the same. In the study by Aghadoost and Khorshidi about 50% of the CONS isolates were resistant to Cotrimoxazole [9]. In the current study, the resistance rates to Cotrimoxaozole were similar in the controls and preservative-free travoprost group and BAK-preserved latanoprost group and were lower than the reported rate of 50%. In the present study more than 70% of all the isolates from both the treatment groups and control group were susceptible to Moxifloxacin, a finding consistent with the findings of the TRUST and ARMOR studies from the U.S [6,7]. No significant effect of BAKpreserved latanoprost or preservative-free travoprost is apparent in the development of resistance against Moxifloxacin since the database of the TRUST and ARMOR studies comprised of folks who were not instilling any medications. In the current study, the isolates from control group had low resistance to Amikacin while isolates from both the treatment groups had no resistant strains. In the study by Mshangila et al resistance to Gentamicin was in the range of 5.6 – 31% and resistance to Tobramycin was in the range of 17.2 – 25.3% [8]. In the study by Aghadoost and Khorshidi, >95% of CONS were rsensitive to Amikacin [9]. These findings are fairly similar to the present study.
Fortunately, in the present study, not a single gram positive bacteria was resistant to Vancomycin (Table 2&3). In the study of Mshangila et al and the study by Aghadoost and Khorshidi, the same finding has been reported [8]. Hence there was no effect of chronic drug instillation irrespective of presence of the preservative BAK on susceptibility to Vancomycin. In this study very few gram negative organisms could be yielded and most of them were susceptible to the first line antibiotics. However, one strain of Escherichia coli was resistant to multiple first line antibiotics and was sensitive to higher antibiotics like Minocycline, Tigecycline, Colistin and Polymixin.
Conclusion
The results of this study suggest that the long-term use of prostaglandin analogues for glaucoma may adversely affect the antibiotic-resistance of indigenous bacterial flora of the eye by increasing the resistance against common antibiotics. In view of the comparatively higher incidence of Methicillin resistant CONS isolates among latanoprost (with BAK) group, long term treatment with BAK-preserved drops may be avoided for the treatment of glaucoma. Further, the tendency of the resistance pattern should be considered in order to prevent such alarming emergence of resistance in bacterial population that may lead to untrea table eye infections during long term treatment with anti-glaucoma drops. However, for a definitive conclusion, a larger study with a larger sample size will be necessary as in this study the differences were not statistically significant owing to scarce culture positivity and a resultant small effective sample size.
References
- Higgins CS, Murtough SM, Williamson E, Hiom SJ, Payne DJ, et al. (2001) Resistance to antibiotics and biocides among non-fermenting Gram-negative bacteria. Clin Microbiol Infect 7(6): 308-315.
- Mayer S, Boos M, Beyer A, Fluit AC, Schmitz FJ (2001) Distribution of the antiseptic resistance genes qacA, qacB and qacC in 497 methicillin-resistant and-susceptible European isolates of Staphylococcus aureus. J Antimicrob Chemother 47(6): 896-897.
- Ohtani S, Shimizu K, Nejima R, Kagaya F, Aihara M, et al. (2017) Conjunctival Bacteria Flora of Glaucoma Patients During Long-Term Administration of Prostaglandin Analog Drops. Invest Ophthalmol Vis Sci 58(10): 3991-3996.
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, et al. (2002) The Ocular Hypertension Treatment Study: Baseline Factors That Predict the Onset of Primary Open-Angle Glaucoma. Arch Ophthalmol 120(6): 714-720.
- Olson R, Donnenfeld E, Bucci FA, Price FW, Raizman M, et al. (2010) Methicillin resistance of Staphylococcus species among health care and nonhealth care workers undergoing cataract surgery. Clin Ophthalmol Auckl NZ 4: 1505-1514.
- Asbell PA, Colby KA, Deng S, McDonnell P, Meisler DM, et al. (2008) Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol 145(6): 951-958.
- Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, et al. (2015) Antibiotic Resistance Among Ocular Pathogens in the United States: Five-Year Results From the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol 133(12): 1445-1454.
- Mshangila B, Paddy M, Kajumbula H, Ateenyi-Agaba C, Kahwa B, et al. (2013) External ocular surface bacterial isolates and their antimicrobial susceptibility patterns among pre-operative cataract patients at Mulago National Hospital in Kampala, Uganda. BMC Ophthalmol 13: 71.
- Aghadoost D, Khorshidi A (2007) Antibiotic Resistance Patterns of Ocular Surface Bacterial Flora. Arch Clin Infect Dis 2(3): 129-132.