Lupine Publishers Group
Lupine Publishers
Research ArticleOpen Access
Anti-Oxidant Effect of Lycium Barbarum Polysaccharides on the Retina of Streptozotocin-induced Diabetes Rats Volume 2 - Issue 5
Jian Guo1, Guoxing Xu1*, Lijin Cui1 and Liying Huang2
- 1Fujian Institute of Ophthalmology, The First Affiliated Hospital of Fujian Medical University, China
- 2The College of pharmacy of Fujian Medical University, China
Received:March 17, 2020; Published:April 07, 2020
Corresponding author: Guoxing Xu, Fujian Institute of Ophthalmology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian Province, 350004, China
DOI: 10.32474/TOOAJ.2020.02.000149
Abstract
Aim: To investigate the effect of Lycium barbarum polysaccharides (LBP) on the oxidation damage of retina of streptozotocininduced diabetes rats.
Methods: The diabetes rats were induced by injection streptozotocin intraperitoneally. LBP was intragastrically administered to the rats of the LBP group. Body weight and blood glucose were measured every four weeks. In the 24th week, the SOD and MDA of retina were measured, the expression of VEGF mRNA of retina was tested by RT-PCR, the ultrastructure was observed by transmission electron microscopy.
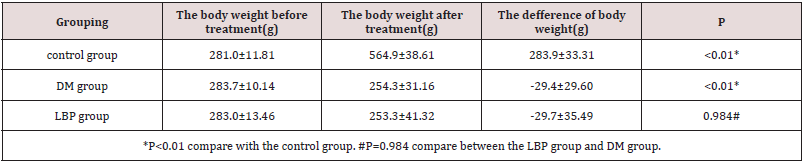
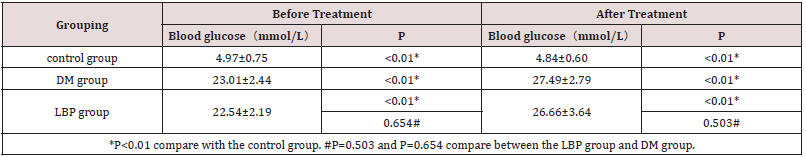
Results: The body weight decreased and the blood glucose increased in DM group and LBP group (P<0.01, VS control group). The body weight and blood glucose is the same level between DM group and LBP group (body weight P=0.503, blood glucose P=0.984). The SOD in DM group decreased and the MDA increased (P<0.05, VS control group). The SOD in LBP group increased and the MDA decreased (P<0.05, VS DM group). The expression of VEGF mRNA was highest in DM group and lowest in control group, and decreased in LBP group (P<0.05, VS DM group). The ultrastructure changes occur in almost all retinal nerve tissues in DM group, as represented by the number and morphology of mitochondria. A small amount of mild mitochondrial changes could be observed in the endochylema of bipolar and Müller cells in LBP group.
Conclusion: LBP can reduce the oxidative damage, alleviate the pathological changes in mitochondria, prevent the apoptosis of nerve cells and block the progression of diseases to the vascular tissue.
Keywords: Lycium Barbarum Polysaccharides; Diabetes; Anti-Oxidant; Diabetic Retinopathy
Introduction
Diabetic retinopathy is one of the most common complications of diabetics and its pathogenesis remains unclear. Studies have shown that mitochondria oxidative damage in retinal nerve cells and neuroglia cell may be the common pathway for the occurrence and development of diabetic retinopathy [1]. Lycium barbarum polysaccharides (LBP) is extracted from the traditional Chinese herbs medicine, medlar, which has a strong anti-oxidant effect [2]. In this study, LBP intervention was used in streptozotocininduced diabetes rats to confirm the anti-oxidant effect through observations of the changes of retina damage.
Materials and Methods
Materials Twenty SPF male SD rats from Shanghai SLAC Laboratory Animal Co. Ltd. were used. No diseases were found in the outer eye and fundus oculi. The rats were raised in the Rodentia SPF Laboratory of the Experimental Animal Center of Fujian Medical University. Blood glucose was measured by the OneTouch Ultra2 Blood Glucose Meter (≤6.75mmol/L). Streptozotocin was dissolved in sterile citric acid–citrate buffer solution. It was prepared in 1% solution before use. LBP (1.05g) which was provided by prof. LY Huang was weighed for dissolution in 17.5ml physiological saline.
Method
Establishment of Streptozotocin-Induced Diabetic Rat Model
The [3] SD rats were randomly divided into the DM group (15 rats) and the control group (5 rats). They were raised adaptively for 1 week, and fasted for 10 hours before modeling. Streptozotocin solution was injected into rats in the DM group via the lower left abdominal cavity(65mg·kg-1). Citric acid–citrate buffer solution of the same quantity was injected into 5 rats in the control group. 72 hours after, blood was taken from the caudal vein to measure blood glucose. When the rats showed a blood glucose level ≥ 16.7mmol·L-1, the measurement of blood glucose level was repeated after 1 week. The rats which blood glucose level ≥ 16.7 mmol·L-1 were picked out for the following experiment.
Experimental Grouping and Observation
Thirteen rats, which passed the modeling, were randomly divided into two groups: the diabetic model group (DM group) and the LBP treatment group (LBP group). Every morning, LBP was intragastrically administered to the rats of the LBP group, whereas physiological saline was intragastrically administrated to the DM group and the control group. Body weight and blood glucose were measured every four weeks.
Observation of SOD and MDA Level of Retina
The rats were weighed and blood glucose were measured after drug administration for 24 weeks. The rats were intraperitoneally injected with napental(60mg·Kg-1) and the eyeballs were removed. The retina was peeled under a ophthalmologic microscope. The retina tissue was added into the pre-cooled physiological saline at 4°C and then cleaned twice. The physiological saline at 4°C was added at a ratio of 1:9. The solution was then homogenized and centrifugated (2000rpm, 10min). The supernatant fluid was retrieved for measurements with a reagent kit, according to the manufacturer’s instructions.
Expression of VEGF mRNA in the Rat Retina Tested by RT-PCR
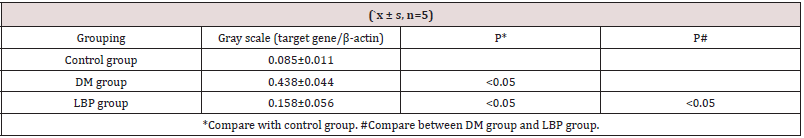
The eyeballs of the rats in each group were taken to peel the retina under a microscope for ophthalmologic operation. The TRIzol method was used to extract total RNA and prepare cDNA by reverse transcription. The cDNA was PCR-amplified, and then agarose gel electrophoresis was performed. A gel imaging system was used for gray scale scanning. The absorbance value was recorded and the VEGF/β -Actin specific value was determined.
Ultrastructure Observation by Transmission Electron Microscopy
Glutaraldehyde myocardial perfusion was performed and the eye ball was rapidly removed. Retina tissue was taken and cut into small rectangular pieces, which were then fixed in glutaraldehyde– paraformaldehyde solution overnight. After fixing in osmic acid– potassium ferrocyanide for cleaning, alcoholized uranyl acetate dye solution was used for En Bloc staining. Alcohol–acetone was used for dehydration in the gradient slope. Epoxide resin embedding medium was used for embedding. The specimens were sliced into ultrathin sections. Uranyl acetate and lead citrate were used for staining; they were observed under a Philips transmission electron microscope.
Statistical Analysis
Data were represented as mean±SD. Statistical analysis was performed by SPSS13.0 statistical software. Statistical differences between groups were analyzed by one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
Results
The difference of Body Weight During Observation
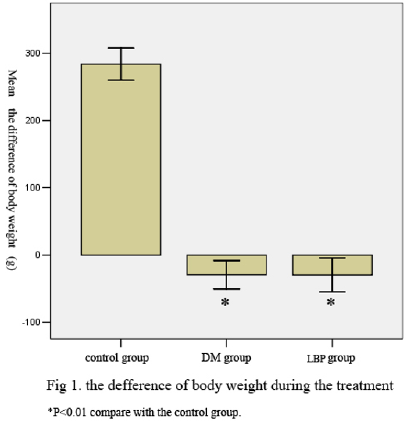
The body weight of the rats increased in control group whereas decreased in DM group and LBP group (P<0.01 VS control group). The change of body weight is the same level between DM group and LBP group (P=0.984) (Table 1, Figure 1).
The Variation of Blood Glucose During Treatment
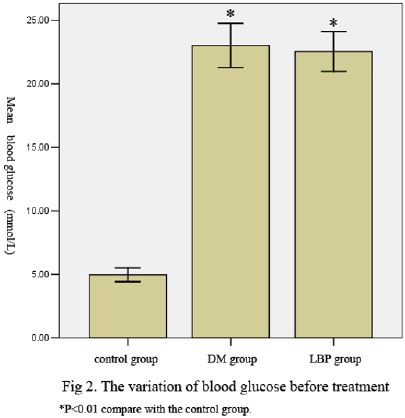
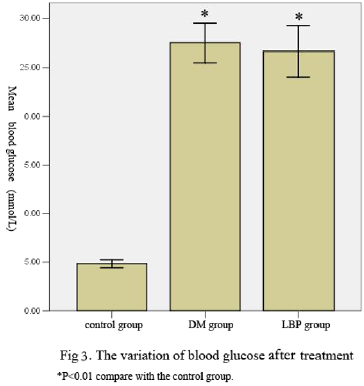
The blood glucose of the rats in DM group and LBP group is higher than the glucose in control group (P<0.01 VS control group). The blood glucose is the same level between DM group and LBP group (P=0.503) (Table 2, Figure 2, 3).
The SOD and MDA Level of Retina
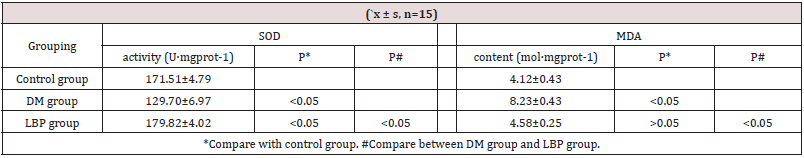
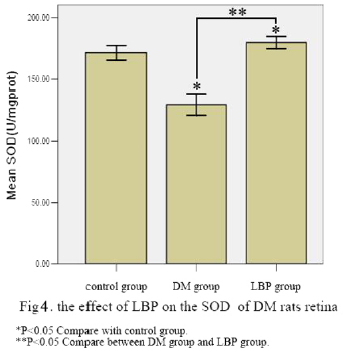
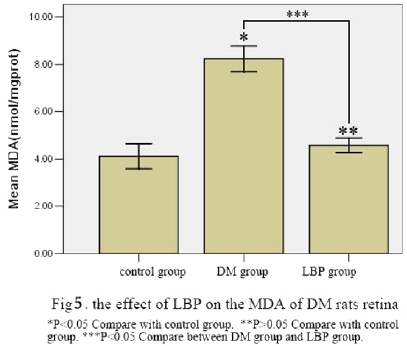
The SOD of retina in DM group decreased and the MDA increased (P<0.05, VS control group). The SOD of retina in LBP group increased and the MDA decreased (P<0.05, VS DM group) (Table 3, Figure 4, 5).
Expression of VEGF mRNA by RT-PCR
The expression of VEGF mRNA in the rat retina was highest in DM group and lowest in control group. The level of VEGF mRNA decreased in LBP group (P<0.05, VS DM group) (Table 4, Figure 6).
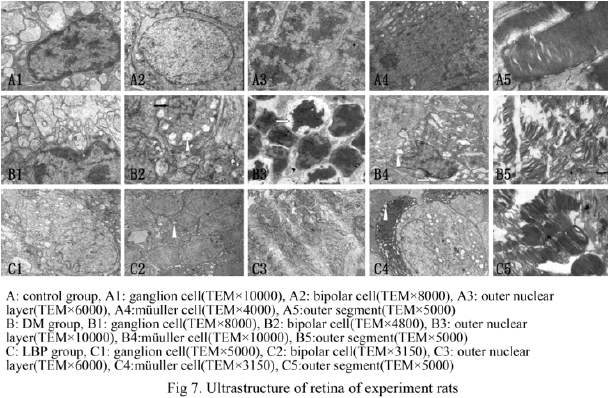
Ultrastructure Observation by Transmission Electron Microscopy
The retinal ultrastructure of the rats in the control group was normal. In the retina of the DM group, the damage of retinal nerve tissue was present in all layer of retina. A large number of huge mitochondria was found in the inner nuclear layer. Crista was tumid, cracked and reduced. Part of the mitochondria was round and vacuole-like. The nuclear membrane was noncontinuous, with an increased number of unevenly distributed heterochromatin. Chromatin was concentrated below the nuclear membrane at different block mass sizes. The perinuclear space was enlarged. The cell nucleus of the partial bipolar cells was shrunk and cracked. Mitochondria and synaptic vesicle were visibly reduced in the neuron axon. The Müller cell volume increased with numerous loose endochylema and vacuoles. Numerous abnormal mitochondria were also found. The perinuclear space was enlarged. The membranous disc of the outer segment was disorderly with an unclear layered structure. The mitochondria in the inner segment ellipsoid was reduced and crista was vacuole-like. The microvilli of Müller cells decreased and exhibited disorderly arrangement. A small number of microvascular pericytes showed heterochromatin condensation and margination, as well as a non-continuous nuclear membrane. No clear change was found in the vascular endothelium cell and basilar membrane. In the LBP treatment group, no obvious abnormality was found in the retinal ganglionic cells and receptor cells. In the bipolar and Müller cells, the partial mitochondria crista became shorter and fewer. No abnormal change was found in the cell nucleus. The inner nuclear layer cells were regularly and tightly arranged. (Figure 7).
Discussion
Diabetic retinopathy is one of the most common eye complications, considered a long-term pathological change in blood capillary level. Recent clinical and animal studies have shown that the pathological changes in retinal nerve tissues occur in the early stage of diabetic. Clinically, abnormal visual functions, including visual electrophysiology, color vision, contrast sensitivity and visual fields, show up before retinal microangiopathy in early stages. Research showed that pathological changes in retinal nerve tissue, such as degeneration and apoptosis of ganglionic cells, photoreceptor cells and neuroglia cells, occurred in the first week in diabetic animal models [4]. Barber believed that diabetic retinopathy is a degenerative disease of the nervous system manifesting in the eyes [5]. Our experimental results had shown that pathological changes in different degrees occur in almost all retinal nerve tissues of streptozotocin-induced diabetic rats in the 24th week, as represented by the change in ganglionic cells, photoreceptor cells, bipolar cells, and neuroglia cells in terms of the number and morphology of mitochondria, increased nucleolus heterochromatin and reduced synaptic vesicle in the axon. Our study revealed obvious and extensive pathological changes in retinal nerve tissues, whereas no clear pathological changes were found in vascular endothelial cells and basilar membrane. Moreover, diabetic retinopathy possibly originated from the pathological changes in nerve tissues.
The pathogenesis of diabetic retinopathy currently remains unclear. Several pathogenic mechanisms have been posited, including the polyol pathway, generation of advanced glycation endoproducts (AGES), protein kinase C (PKC) and aminohexose pathway. Recent studies have shown that the generation of a large number of reactive oxygen species (ROS) induced by high glucose may be the common pathogenic factor of many kinds of chronic diabetic complications, including diabetic retinopathy [6]. A uniform pathogenic mechanism of diabetic complications was proposed in 2005 [7], the authors argued that the pathogenic mechanism of all diabetic complications is a unique pathway, the electron transfer chain of mitochondria induced by high glucose produces excessive superoxide. The pathogenic pathway determined by previous studies, including the polyol pathway, PKC activation, generation of AGE precursors in cells and activation of amidohexose pathway, resulted from the increase in mitochondrial ROS. ROS consists of a series of functional groups, such as O-, H2O2 and NO. Many pathways can generate ROS while diabetic occurs. For example, the glycosylation and electron transfer chain of mitochondria can be activated. AGES, insulin and angiotensin II can induce ROS by activating NADPH oxidase combined with epicyte [8,9]. The study of diabetic renal disease indicated that the inhibition of NADPH oxidase can prevent AGE-induced renal damage in diabetic patients [10]. Under normal conditions, the antioxidant system in organisms can eliminate the oxidative substances produced by metabolism, and maintains the dynamic balance of the internal environment. For example, superoxide dismutase (SOD) can eliminate all kinds of oxygen radicals. When an organism produces more oxygen radicals beyond its antioxidant ability, ROS damages its tissues and organs. These attack the mitochondrial membrane, resulting in apoptosis in the mitochondrial pathways [11]. Excessive ROS also activates the action pathways of all known diabetic complications, including the polyol pathway, PKC, AGES precursors and amidohexose pathway, thereby inducing diabetic complications in multiple organs [12]. Malondialdehyde (MDA) is a lipid peroxidation product. It affects the electron transfer chain of mitochondria and key enzyme activity, aggravating the oxidative damage of cells. Therefore, MDA is also one of the key indicators of oxidative reaction in an organism. In our experiment, the SOD activity of diabetic rats induced by streptozocin decreased, whereas MDA increased. This finding confirms that oxidative stress has an important effect on the pathological changes in the retinal nerve tissues of diabetic rats. The ultrastructure of diabetic rats retina showed that the most prominent changes in retinal neurons and neuroglia cells are the changes of mitochondria in the number, size and morphology. Several huge mitochondria can be found in the inner nuclear layer and inner molecular layer. The number of mitochondria in axons, photoreceptors and other positions decreased, with short, cracked and decreased cristae. Part of the cristae was beakerlike. In severe cases, the entire mitochondria exhibited vacuolar changes, indicating the importance of mitochondrial change in the pathological mechanism of diabetic retinopathy. Lycium barbarum polysaccharides (LBP) is extracted from the Chinese traditional herbs medicine, Ningxia medlar. Modern pharmacological studies found that LBP can remove redundant free radicals in the body [13], improve the activity of antioxidant enzymes [14,15] and increase the survival rate and promote the growth of rat retinal ganglion cells [16]. In our study, no significant difference in blood glucose level was found between the LBP group and the DM group before treatment, showing that the two groups were comparable. For blood glucose and body weight after treatment, no significant difference was also found between the two groups. These findings show that LBP could not reduce the blood glucose of animals. This result differs from that derived by Liu Ping [17]. The method they adopted was low dosage combined with high amounts of fat and sugar. The blood glucose of the modeled animal was 13.43 ± 1.36 mmol·L-1. Our study adopted the high-dosage streptozocin simple modeling method. The blood glucose of the diabetic model rats were more than 20 mmol·L-1. Therefore, our research can rule out the possibility of LBP indirectly curing diabetic retinopathy by reducing blood glucose. Significant pathological changes were not found in the gangliocyte and photoreceptor cell of the LBP group. A small amount of mild mitochondrial change could be observed in the endochylema of bipolar and Müller cells. This change manifested as the shortening and reduction in the number of cristae. SOD activity of the retina visibly increased and MDA decreased, indicating that LBP could alleviate the diabetic-induced pathological changes in retinal nerve tissues by reducing the oxidative damage caused by mitochondria pathway. The down-regulated VEGF expression showed that antioxidant therapy early can control the progression of retinal microangiopathy. However, the relation between the retinal nerve tissue pathological change and microangiopathy is unclear.
Thus, the early retinopathy of diabetic mainly occurs in the nerve cell and neuroglia cell by oxidative damage of mitochondria pathway. LBP can reduce the oxidative damage, alleviate the pathological changes in mitochondria, prevent the apoptosis of nerve cells and block the progression of diseases to the vascular tissue. LBP can be used in the early prevention and cure of diabetic retinal nerve pathological changes.
Foundation Item
1. National Natural Science Foundation of China(No.81770948).
2. The traditional chinese medicine research project of Fujian Province (No.wzzyb0905).
3. Fujian provincial science and technology innovation leadership talent foundation (No.2016B011)
4. Fund project for training young and middle-aged key talents of Fujian Provincial Health Committee(2016-ZQN-37).
References
- Nishikawa T (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404(6): 787-790.
- Liu CJ, Jiang B, Liu L, Fan SD (2009) Optimization of the Extraction Technology and Antioxidative Activity of Polysaccharide from Fructus Lycii. Lishizhen Medicine and Materia Medica Research 20(3): 661-663.
- Lin BQ, Zhou JY, Ma Y, Deng YJ, Zheng CJ, et al. (2011) Preventive effect of danhong huayu koufuye on diabetic retinopathy in rats. Int J Ophthalmol 4(6): 599-604.
- Martin PM, Roon P, Ells TK, Ganapathy V, Smith SB (2004) Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthamol Vis Sci 45(9): 3330-3336.
- Barber AJ (2003) A new view of diabetic retinoPathy: A neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 27(2): 283-290.
- Hideaki Kaneto, Naoto Katakami, Munehide Matsuhisa, Taka aki Matsuoka (2010) Role of Reactive Oxygen Species in the Progression of Type 2 Diabetes and Atherosclerosis. Mediators Inflamm 453892.
- Brownlee M (2005) The pathobiology of diabetic complications:a unifying mechanism. Diabetes 54: 1615-1625.
- Ojima A, Matsui T, Maeda S, Takeuchi M, Yamagishi S (2012) Glucose-dependent Insulinotropic Polypeptide (GIP) Inhibits Signaling Pathways of Advanced Glycation End Products (AGEs) in Endothelial Cells via its Antioxidative Properties. Horm Metab Res 44(7): 501-505.
- Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, et al. (2009) RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol 20(4): 742-752.
- Bonke VT, Thorpe SR, Coughlan MT, Fukami K, Yap FV, et al. (2008) Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes 57(2): 460-469.
- Bing Lin, Zhuo Chen, Yufang Xu, Huanying Zhang, Jianwen Liu (2011) 7-b, a novel amonafide analogue, cause growth inhibition and apoptosis in Raji cells via a ROS-mediated mitochondrial pathway. Leuk Res 35(5): 646-656.
- Gang Xi, Xinchun Shen, Maile LA, Wai C, Gollahon K, et al. (2012) Hyperglycemia enhances IGF-I-stimulated Src activation via increasing Nox4-derived reactive oxygen species in a PKCζ-dependent manner in vascular smooth muscle cells. Diabetes 61(1): 104-113.
- Quan Liu, Yanqing Li, Li Hu, Dehui Wang (2011) Lycium barbarum Polysaccharides Attenuate Cisplatin-Induced Hair Cell Loss in Rat Cochlear Organotypic Cultures. Int J Mol Sci 12(12): 8982-8992.
- Xiaozhong Shan, Junlai Zhou, Tao Ma, Qiongxia Chai (2011) Lycium barbarum Polysaccharides Reduce Exercise-Induced Oxidative Stress. Int J Mol Sci 12(2): 1081-1088.
- Cheng D, Kong H (2011) The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules 17 (3): 2542-2550.
- Yang M, Gao N, Zhao Y, Liu LX, Lu XJ (2011) Protective effect of Lycium barbarum polysaccharide on retinal ganglion cells in vitro. Int J Ophthalmol 4(4):377-379.
- Liu Ping, He Langjie (2008) Effect of Lycium barbarum polysaccharide on glucose and lipid metabolism in diabetic rats. Journal of Ningxia Medical University 30(4): 427-428.