Lupine Publishers Group
Lupine Publishers
Review ArticleOpen Access
A Review of Metabolic Sensors in Glaucoma Volume 3 - Issue 1
Powell S MB1, Irnaten M1 and O Brien CJ MD1,2*
- 1Department of Ophthalmology, Mater Misericordiae University Hospital, Ireland
- 2School of Medicine and Medical Science, University College Dublin, Ireland
Received:November 07, 2020; Published:November 13, 2020
Corresponding author: Colm O’Brien, Institute of Ophthalmology, School of Medicine and Medical Science, University College Dublin, Dublin, Ireland
DOI: 10.32474/TOOAJ.2020.03.000155
Abstract
Glaucoma is the second leading cause of irreversible blindness worldwide. It is a multifactorial, progressive, chronic optic neuropathy that is characterized by loss of retinal ganglion cells (RGC) and optic nerve head (ONH) cupping including extra cellular matrix (ECM) remodelling and fibrosis at the lamina cribrosa (LC). Clinically this results in chronic, progressive peripheral visual field loss. The pathogenesis of glaucoma is not yet fully understood. Therefore, there is an urgent need to identify and target the underlying mechanisms governing ECM remodelling of the LC, in order to stop the progressive, chronic damage to the LC/ONH and irreversible visual field loss. This review identifies and examines some of the key metabolic processes and cellular sensors involved in the pathogenesis of ECM fibrosis in general but herein specifically in glaucoma, including mitochondrial dysfunction and adenosine monophosphate activated protein kinase (AMPK) upregulation. Furthermore, the development of novel therapeutics such as nicotinamide (NAM) and metformin are discussed as promising potential future therapeutic options for glaucoma.
Keywords: Glaucoma; Fibrosis; Extracellular Matrix (Ecm) Mitochondrial Dysfunction; Adenosine Monophosphate Activated Protein Kinase (Ampk); Nicotinamide (Nam); Metformin
Introduction
Glaucoma is the second leading cause of irreversible blindness worldwide. More than 67 million people are affected by glaucoma, which has a global prevalence of 3.5% in persons over the age of forty years [1]. Glaucoma is heterogenous group of progressive optic neuropathy disorders resulting in loss of peripheral field vision. The primary risk factor associated with the development of glaucoma is elevated intraocular pressure (IOP). Currently, lowering IOP is the only pharmacological treatment available for managing glaucoma [2]. However, there is a subset of glaucoma patients with normal IOP. Studies have shown that normal tension glaucoma (NTG) accounts for approximately 30% of all patients diagnosed with glaucoma [1]. This poses a significant treatment obstacle, as the efficacy of IOP lowering drops is highly variable among this cohort of NTG patients [3]. The major clinical presentations of glaucoma include cupping and pallor of the ONH. Glaucoma is characterized by loss of retinal ganglion cells (RGC) and optic nerve head (ONH) cupping [3]. This dysfunction occurs in the cells of the lamina cribrosa (LC) region, which is a three-dimensional (3D), fenestrated, mesh like structure located in the ONH. Under normal circumstances, the LC functions as a structural support to unmyelinated RGC axons as they pass through posteriorly, before exiting the eye to the brain, becoming the optic nerve [4,5]. The LC is a biomechanically weaker structure when compared to the rest of the sclera and is thereby the putative site of RGC damage in glaucoma. Laminar cupping is connective tissue-based, with the LC progressively moving posteriorly and excavating beneath the anterior sclera canal leading to remodelling of extracellular matrix (ECM), stiffness and consequently fibrosis of the LC connective tissue in the ONH [3], which is driven by profibrotic growth factors such as transforming growth factor- beta (TGFβ) [6] and backward bowing. This results in optic nerve axon compression, axonal loss and, ultimately, chronic, progressive peripheral visual field defects [7].
The cellular mechanisms involved in glaucoma are not fully understood. Understanding these processes will lead to new methods of preventing chronic glaucomatous vision loss when conventional IOP-lowering treatments either fail to prevent progression (up to 20% of glaucoma patients continue to progress to blindness) [5], or adequate target pressures cannot be achieved due to ineffectiveness of or poor tolerance to medication. Thus, there is an urgent need to identify and target the underlying mechanisms of metabolism governing remodelling of the LC, in order to stop the progressive, chronic damage to the LC/ONH and irreversible visual field loss. This review aims to identify and examine some of the key metabolic processes and cellular sensors involved in the pathogenesis of fibrosis in glaucoma and investigate potential therapeutic options.
Fibrosis
Whilst the pathogenesis of glaucoma has yet to be fully elucidated, it is known that connective tissue fibrosis is one of the major pathological drivers of the disease. The term fibrosis is defined as the process of unchecked wound healing [8]. Fibrosis is involved in the pathogenesis of many systemic diseases involving multiple tissue and organ systems such as the heart, liver, lungs and kidneys. Fibrosis results in chronic, progressive, pathological destruction of tissue and organ function [9,10]. The normal wound healing response of tissues to damage is a highly complex process, involving activation of the coagulation cascade, inflammation, angiogenesis, cellular proliferation and tissue remodelling [10]. Activated fibroblasts, known as collagen producing myofibroblasts that are highly contractile, drive the wound healing response to an acute injury by remodelling extracellular matrix (ECM) resulting in the restoration of tissue integrity and previously damaged parenchymal cells [11-12]. This normal wound healing process becomes dysregulated and uncontrolled in states of chronic inflammation, recurrent or repetitive injury or chronic hypoxia, resulting in tissue fibrosis. The ECM is composed primarily of fibrous proteins (primarily collagens, glycoproteins such as fibronectins and vitronectins, and laminins) and proteoglycans [11]. ECM provides structural and biochemical support to cells and tissues in multicellular organisms, in addition to functioning biochemically as a substrate for cell adhesion, growth and differentiation. Thus, ECM is essential for normal connective tissue structure, architecture, differentiation, and homeostasis [14].
Fibrosis in Glaucoma
Fibrosis is characterized by a pathological deposition and accumulation of ECM by myofibroblasts. It is already known that there is an excessive accumulation of ECM in glaucomatous LC tissue, trabecular meshwork (TM) cells and Schlemm’s Canal [11], which results in ONH remodelling and damage. In response to elevated IOP, the ECM genes of glaucomatous LC cells become structurally stiffer and less compliant than normal LC cells, thereby acting as a pro-fibrotic driver of disease [12-15]. ECM stiffness is also a hallmark of tissue ageing. It is known that aged tissues display decreased levels of proteins cadherin and catenin, thin basement membranes and apoptotic resistant, senescent fibroblasts which release fibronectin, matrix metalloproteinases (MMPs) and pro-fibrotic cytokines [13]. Furthermore, aged tissues display a defective upregulation of cross-linked collagen fibres which drives tissue stiffness and rigidity [12], and older age is a significant risk factor for glaucoma. Several other pro-fibrotic mediators are overexpressed in glaucoma, including cytokine transforming growth factor ß (TGFß) and thrombospondin-1 (TSP-1) [3]. TGFß, which is present in the aqueous humour, is a pro-fibrotic cytokine that induces the differentiation of fibroblasts to their collagen secreting form, myofibroblasts. TGFß controls ECM synthesis and activates ECM through the signal transducer family Smad proteins [16,17]. When activated, Smad proteins translocate to the nucleus where they function to regulate gene transcription [13,15]. Our group has shown that LC cells from glaucoma donors [9] have many characteristics of myofibroblasts including the expression of α-SMA, and a marked expression of pro-fibrotic ECM genes and proteins (e.g. collagen 1A1, periostin, fibronectin) upon stimulation with TGFβ [10], cyclic stretch11, and oxidative stress [12]. In another study, we found an increase of F-actin stress fibres (indicating enhanced cellular reorganisation) and increased substrate stiffness elicits a myofibroblastic phenotype in human LC cells [13,14].
Mitochondrial Dysfunction in Glaucoma
Mitochondria, commonly known as the ‘powerhouse’ of the eukaryotic cell, are cellular organelles responsible for the energy production of the cell, and the regulation of cellular metabolism [18]. Mitochondria produce adenosine triphosphate (ATP) primarily through oxidative phosphorylation (OXPHOS), in addition to glycolysis and the Krebs cycle (citric acid cycle). Energy disruption, particularly mitochondrial dysfunction, which results in the toxic accumulation of reactive oxygen species (ROS) within cells has been studied in several disease models over the past decade including cancer, diabetes, and neurodegenerative diseases including Alzheimer’s Disease, muscular dystrophy [19]. A number of studies have examined the role of mitochondrial dysfunction in both human and animal models of glaucoma.
Mitochondrial Dysfunction Studied in Glaucomatous TM Cells
TM cells in glaucomatous human POAG patient eyes were shown to have dysfunctional and defective mitochondria, which resulted in uncontrolled, elevated IOP. These TM cells were more also more vulnerable to Ca2+stress when compared to healthy aged, matched controls, and authors postulated that this vulnerability contributes to sustained, chronic rise in IOP [20]. A recent study of a cohort of ocular hypertensive patients with a longstanding history of raised IOP (‘susceptible’), but whom had never developed glaucoma was performed. Lascaratos etl al found that the ‘susceptible’ patient cohort with ocular hypertension demonstrated both higher levels of mitochondria and ADP phosphorylation, and were better able to withstand and manage cellular stresses such as oxidative stress and excess calcium, versus patients with glaucoma and aged matched controls [21]. They concluded that enhanced mitochondrial activity in a systemic capacity confers protection to the development of RGC damage, ON damage and ultimately the development of glaucoma in addition to identifying mitochondria as a disease biomarker [21]. It has been shown via electron microscope analysis that mitochondria found in the ON in glaucomatous eyes are notably smaller and fewer when compared to normal age matched eyes [22,23] Furthermore, studies have demonstrated a reduced number of cristae within mitochondria, which means cells contain less tools to perform effective OXPHOS. Consequently, cells possess lower and deficient energy capacity [19,23]. This has a direct downstream effect on axonal survival in circumstances such as glucose depletion. Axons require functional mitochondria in order to survive on lactate, which normally bypasses the process of glycolysis on conversion to pyruvate. However, it has been demonstrated in glaucomatous optic nerves that mitochondria are not capable of effective regeneration, thereby leading to downstream axonal death [22].
Mitochondrial Dysfunction Studied in Glaucomatous Retinae
In glaucoma, defective mitochondrial function is associated with both disease susceptibility and disease resistance [19]. In fact, in vivo studies of mice have demonstrated mitochondrial dysfunction to be one of the primary detectable features of stressed RGC’s in response to elevated IOP [24]. Inman et al. demonstrated that defective mitochondrial DNA and metabolic dysregulation occur prior to evidence of neurodegeneration [19]. It is established that RGC’s require a large quantity of ATP due to the dense axonal volume of mitochondria surrounding the OHN. Mitochondrial dysfunction in RGCs, which results in defective cellular repair process thereby enhances their susceptibility to apoptosis and subsequent glaucomatous pathogenesis [25]. Harun et al. studied DBA/2J mice models and showed that monocarboylate transporters (MCT), which function as lactate and ketone transportation molecules, are under expressed in glaucomatous retinae, and result in decreased ATP production [26] Mice received an injection of MCT2 (AAV2:MCT2) in order to restore MCT2 to normal cellular levels, and it was shown that RGCs were preserved in this group of mice. Importantly, following the induction of MCT2 overexpression in DBA/2 J retinae via AAV2:MCT2 injection, mitochondrial function in the retinae of these mice improved. Additionally, an increase in RGC density and an enhancement of energy homeostasis were also noted in DBA/2J mice versus the untreated cohort. This study demonstrates both the neuroprotective effect of MCT2 on glaucomatous RGCs, in addition to highlighting the potential therapeutic benefit enhanced cellular energy input may have in glaucoma treatment in the future [26].
Mitochondrial Dysfunction in Blood Analyses of Glaucoma Patients
Mitochondrial dysfunction in lymphocytes of a cohort of primary open angle glaucoma (POAG) patients has been examined [27]. Lee et al. analysed ATP production and cellular respiration in POAG patients and found that POAG lymphoblasts displayed decreased levels of complex 1-driven ATP synthesis and complex-1 driven maximal respiration when compared to controls. Complex 1 (NADH: ubiquinone oxidoreductase), whose role is electron transport, is the biggest enzymatic complex of the mitochondrial respiratory chain [28]. There was no difference in complex-2 linked respiration between the two groups, nor was there any difference in ATP production when cells were grown on galactose media (thereby reliant on mitochondria OXPHOS [27]). Similarly, another recent study conducted a comparative analysis of mitochondrial OXPHOS complex-1 dysfunction in patients with Leber Hereditary Optic Neuropathy (LHON) when compared to POAG patients. LHON is a mitochondrially inherited disorder involving complex-1 mutations, and is characterized by the quick, aggressive and irreversible loss of RGCs [29]. POAG, LHON and normal lymphoblasts were cultured on galactose media, and the growth rates of the groups were examined. POAG lymphoblasts and LHON lymphoblasts grew 1.47 and 2.35 times slower than control lymphoblasts. Furthermore, when compared to controls, POAG lymphoblasts demonstrated an 18% reduction in complex-1 activity, versus a 29% decreased in LHON lymphoblasts. Finally, when complex-1 ATP synthesis between the groups was compared to control samples, a 19% reduction was noted among the POAG group whereas a 17% decrease was observed in LHON patients [29]. This study demonstrates OXPHOS impairment in both POAG and LHON patients and proposes that the milder dysfunction of the POAG group versus LHON patients might reflect a less aggressive nature and progression of glaucoma. Finally, this study highlights the potential role of restoring mitochondrial function as a promising therapeutic target for diseases characterized by mitochondrial dysfunction, including glaucoma. A study using Gene-Set Analyses was conducted on a cohort of POAG and NTG patients to assess mitochondrial gene associations [30]. Khawaja et al. identified a strong association between POAG and lipid metabolism pathways (P<0.002) and butanoate metabolism, which is a carbohydrate metabolism pathway (P<0.004). This study demonstrates an important role of lipid and carbohydrate metabolism in the disease pathogenesis of POAG.
Mitochondrial Dysfunction in Glaucomatous LC Cells
Our group has demonstrated that glaucoma LC cells proliferate at a higher rate than normal LC cells [15] and show mitochondrial dysfunction [16]. Kamel et al. conducted a detailed mitochondrial bioenergetic assessment on normal and glaucoma human LC cells [31] which revealed significantly abnormal mitochondrial respiratory bioenergetic function in human glaucoma LC cells when compared to controls. Decreased ATP production at basal levels, reduced OXPHOS and increased glycolysis were observed. Furthermore, MCT1 (OXPHOS marker), MCT4 (glycolysis marker), MTHFD2 (folate-mediated one-carbon metabolism marker), and GLS2 (glutaminolysis marker) were overexpressed in the glaucoma patient cohort. Thus, the findings of this study indicate that glaucoma cells undergo a process of ‘metabolic reprogramming’, and essentially switch from OXPHOS to aerobic glycolysis. This phenomenon is known as ‘the Warburg effect’, and it is a well-known, longstanding feature of neoplastic cells and cancer associated fibroblasts (CAF) [32,33].
The Role of Nicotinamide (NAM) in Glaucoma
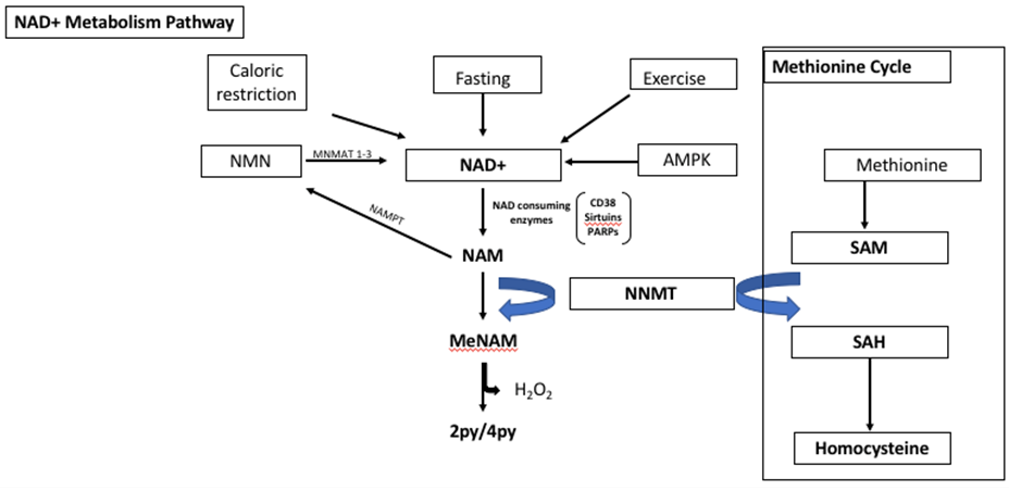
NAD+ is activated by caloric restriction, fasting, exercise and AMPK. NAD+ is metabolised to nicotinamide (NAM) by NAD+ consuming enzymes such as CD38, Sirtuins and poly adenosine diphosphate ribose polymerase (PARPs). NAM is a water-soluble form of niacin (Vitamin B3). NAM can be converted to nicotinamide mononucleotide (NMN) by nicotinamide phosphoribosyl transferase (NAMPT) and then to NAD+ by nicotinamide mononucleotide adeneylytransferase 1 and 3 (NMNAT 1-3), via the salvage and pathway. Nicotinamide N-Methyl Transferase (NNMT) catalyses the methylation of NAM to N-methyl-nicotinamide (metNAM), which is subsequently metabolised further to N1-methyl-2-pyridine-5-carboxamide (2py) or N1-methyl-4-ppyridone-3-carboxamide (4py) utilizing the methyl donor S-adenosyl methionine (SAM) from the methionine cycle. 2py and 4py are the primary metabolites of NAM. NAM in reversing or halting disease pathogenesis. For example, NAM supplementation studied in obesity models in mice resulted in reduced oxidative stress and inflammation as well as repleted glycogen storage capacity [34]. NAM supplementation however, had no direct effect on lifespan, rather it enhanced healthspan [34].
The potential role of bioavailable NAM as a drug therapeutic target has been studies in animal models of glaucoma [24]. Williams, et al. studied DBA/2J mice models and showed that nicotinamide has a neuroprotective role in glaucoma. In glaucoma, reduced levels of NAD+, mitochondrial damage and glutathione depletion result in fragile, vulnerable RGCs. A single molecule of low dose NAM (NAMLo), high dose NAM (NAMHi) and finally NMNAT gene therapy was administered [24]. NAMLo mice demonstrated neuronal protection against glaucoma development, with no mitchondrial dysfunction, and decreased levels of DNA damage observed. NAMLo had no impact on IOP levels. 93% of NAMHi mice displayed no cellular signs optic nerve damage. Furthermore, NAMHi had a protective effect on IOP levels. This study implies that NAMHi confers not only a protective effect against glaucoma development but a protective effect on cellular types other than RGCs [24]. Another recent study led by Hiu, et al. demonstrated an improvement of inner retinal function in glaucoma patients following the administration of NAM supplementation [35]. They conducted a randomised control trial of fifty-seven patients with known, well controlled glaucoma. Patients were commenced on a regimen of NAM over three months. (1.5g/day for 6/52 followed by 3.0g/day for 6/52). Photopic negative response (PhNR) which is an electroretinogram (ERG) parameter was used as the measurement of inner retinal function (Saturated PhNR amplitude = Vmax). The study found that Vmax improved by 14.8%(p=0.02) in patients receiving NAM versus 5.2% (p=0.27) in the placebo group. When comparing visual field (VF) mean deviation (MD) 27% of patients on NAM improved by ≥1dB, and additionally fewer patients on NAM demonstrated any worsening of VF (4%), versus the control group (p=0.02). This study further highlights a promising potential for NAM supplementation in the treatment of glaucoma, although it is clear that further studies must be performed on a larger scale in order to fully elucidate the effectiveness and determine the long term safety of NAM in glaucoma therapy.
Nicotinamide adenine dinucleotide (NAD+) is found in the cytoplasm, mitochondria and nuclei of eukaryotic cells [36]. NAD+ plays a central role in the regulation of several biological processes including metabolism, cell signalling, DNA repair and cellular longevity [37]. Reduction in NAD+ levels have been associated with several age-related diseases, including carcinoma, cardiovascular disease (CAD), neurodegenerative and metabolic disorders [38]. NMNAT2, an enzyme producing NAD is essential for RGCs, healthy axons and in the prevention of axonal degeneration [39]. NMNAT2 is a survival factor necessary for axon survival. NMNAT2 null mice demonstrate truncated RGC axons and have no optic tract. Furthermore, siRNA knockdown of NMNAT2 results in the degeneration of neurons despite the absence of injury [39]. Mice were injected with AAV2.2 which contained the Nmnat1 gene. >70% of mice treated with this vector were found to have no clinical or pathological signs of optic nerve damage. Finally, mice were treated with both Nmat1 gene in addition to NAMLo. 84% of mice receiving this combination therapy were free of any glaucomatous damage, therefore it is postulated that a combination therapy confers additional protection and reduces vulnerability of the RGC cells of the LC to glaucomatous changes [24]. A number of studies have examined the potential therapeutic role of NAM in Parkinsons Disease (PD) patients. One mouse model study conducted by Harrison et al. showed that NAM administration improved locomotor responses and lessened dopamine depletion, thereby demonstrating a neuroprotective role for NAM [40]. Conversely, however, other studies have shown that NAM is associated with PD and exacerbates neurodegeneration [41]. Interestingly, levels of NNMT and metNAM were found to be raised in PD brains [42]. These conflicting reports warrant further analyses and studies in order to accurately elucidate the role of NAM in PD and neurodegenerative diseases.
Nicotinamide N-Methyl Transferase (NNMT)
Nicotinamide N-Methyl Transferase (NNMT) is a cytosolic enzyme that is expressed at the highest level in the liver [40]. NNMT catalyses the methylation of NAM to N-methyl-nicotinamide (metNAM), which is subsequently metabolised further to N1-methyl-2-pyridine-5-carboxamide (2py) or N1-methyl-4-ppyridone-3-carboxamide (4py) utilizing the methyl donor S-adenosyl methionine (SAM) [37,40] [Figure 1]. NNMT eliminates NAM from the NAD+ synthesis pathway, resulting in depleted levels of NAD+. NNMT is expressed in several tissues such as the heart, brain, kidney and muscle, and its upregulation and overexpression has been linked with various disease pathogenesis such as cancer, metabolic, neurodegenerative and inflammatory disorders [37,43]. Furthermore, there have been several associations between high levels of SAM, which is a homocysteine precursor, and insulin resistance and cardiovascular disease (CAD) [44,45]. Importantly, high levels of serum NNMT and NAM are associated with increased severity of CAD [46]. In mice models, techniques such as genetic knockdown and drug inhibition of NNMT was found to be protective against obesity and type two diabetes [47]. Authors treated mice with a molecular analogue of NAD (JBSNF000088), which inhibited NNMT activity. Mice treated with the analogue displayed lowered body weight, an improvement to normal glucose tolerance and an improvement in insulin sensitivity [47].
AMPK as a Sensor of Cellular Stress
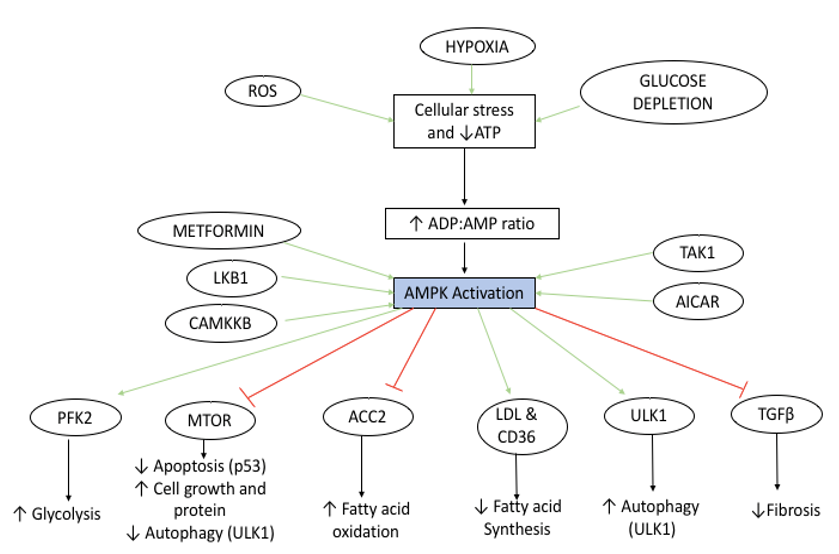
AMPK is activated by a variety of stimuli including an increase in ADP:AMP ratio that occurs due to cellular stress (reactive oxygen species (ROS), hypoxia and glucose depletion. AMPK is also activated by serine threonine 11 (STK1 or LKB1), Calcium/ Calmodulin-dependent protein kinase kinase β (CAMMKβ) and TAK kinase as well as the adenosine analogue 5-aminoimidazole- 4-carbozamide riboside (AICAR) and therapeutic biguanide metformin. Downstream effects of AMPK activation include an increase in fatty acid oxidation (Low Density Lipoprotein (LDL) and Cluster of Differentiation (CD36) activation), autophagy (Unc-51 Like Autophagy Activating Kinase 1 (ULK1) activation) and glycolysis (Phosphofructokinase 2 (PFK2) activation). AMPK inhibits acetyl-CoA carboxylase 2 (ACC2) and transforming growth factor β (TGFβ) signalling, resulting in both decreased fatty acid synthesis and decreased fibrosis, respectively. AMPK also inhibits the mammalian target of rapamycin (mTOR) signalling pathway by phosphorylation, and this results in apoptosis, cell growth and signalling ana a reduction in cellular autophagy. The overall downstream consequence of AMPK activation is to increase catabolic processes and inhibit anabolic metabolism. The main sensor of cellular energy status in virtually all cells is the adenosine monophosphate activated protein kinase (AMPK), which is highly conserved across all eukaryotic species. In general, AMPK is activated in response to energy stress by sensing increases in AMP:ATP and ADP:ATP ratios and restores energy balance by inhibiting anabolic processes that consume ATP, while promoting catabolic processes that generate ATP. AMPK is activated by the phosphorylation of Thr172. This occurs in states of metabolic stress such as ischaemia, glucose/nutrient starvation, hypoxia or depleted ATP status, making AMPK an excellent indicator and sensor of metabolic energy status within the cell [14,15]. In fact, AMPK controls nearly all cellular functions, including mitochondrial stasis, cell growth and autophagy and is thought of as the ‘master regulator’ of cellular energy status [48,49]. [Figure 2] Moreover, the activity of AMPK is extensively regulated by multiple upstream signals, thus making AMPK a central node exploited by cells to coordinate their metabolism with specific energy demands [18].
Figure 2: Schematic overview of the Adenosine Monophosphate Protein Kinase (AMPK) Activation and downstream signaling pathway.
AMPK is Upregulated in Glaucoma
A recent study on mice models identified AMPK as a central regulator of ECM and cytoskeletal arrangement in trabecular meshwork cells. Chatterjee et al. studied AMPK levels in ECM of human TM cells and examined the effect of AMPKa2 deletion on IOP and aqueous humour drainage [50]. They found that AMPKa1 and AMPKa2 are expressed in TM cells. Furthermore, AMPKa2-null mice demonstrated higher IOP (6%) and dysfunctional aqueous humour clearance versus WT controls [50]. There were no morphological differences in angle anatomy between the groups. Similarly, another study performed on DBA/2J mice found that AMPK was upregulated in the ON and retinae of glaucomatous eyes. They postulated that this activation drives the NF-kB pathway which elicits a pro inflammatory mechanism [51]. The authors examined the effect of a ketogenic diet and showed that a ketogenic diet both inhibited AMPK activation and stimulated Hydroxycarboxylic acid (HCAR1-ARBB2) which in turn downregulates the NLRP3 inflammasome. The overall effect of this is the reduction and inhibition of the pro inflammatory cascade. They concluded that a ketogenic diet confers a protective mechanism in the pathogenesis of glaucoma and suggests its potential as a therapeutic biomarker for neurodegenerative diseases in general [51]. It is well established that alteration of ADP:AMP ratio secondary to cellular glucose deprivation triggers the activation of AMPK by a canonical mechanism [49]. Recent studies have shown that AMPK can also be activated by glucose starvation by a pathway independent of any change in the ADP:AMP ratio. A decrease in levels of both Fructose 1,6 bisphosphate (FBP) and extracellular glucose have been identified as the key sensors by which AMPK is activated. The AMPK activation complex is formed at the surface of the lysosome and consists of v-ATPase, AXIN, LKB1, p18/LAMTOR1 and AMPK [52]. When aldolase is knocked down, AMPK is still activated even in cells with sufficient glucose stores, whereas the D34S aldolase mutant, which remains bound to FBP results in AMPK blockade [52].
AMPK as a Driver of Systemic Disease
AMPK is dysregulated in various disease pathogeneses, and most of the research to date has been centred around its involvement with carcinoma. Genetic analyses have shown that, under normal cellular circumstances, AMPK displays tumour suppressive properties. However, upon development of carcinomatous cells, AMPK becomes an important driver for tumorigenesis [53]. In particular, AMPK related kinase 5 (ARK5) (also known as NUAK 1) is a member of the AMPK class of serine/threonine protein kinases whose role as a cancer biomarker has been vastly studied [54]. ARK5 has been strongly associated with increased tumour invasiveness [55], tumour growth, metastasis [56], cellular survival in hypoxic and nutrient-deprived environments (austerity) [57], and epithelial mesenchymal transformation (EMT) in a wide range of human cancers [54,59]. Furthermore, there is evidence that ARK5 is involved in the pathogenesis of fibrosis and, importantly, that it is intrinsically linked with TGFß1 [60]. Brennan et al. studied renal epithelial cells of diabetic nephropathy patients and examined the relationship between ARK5 and TGFβ using next generation sequencing. They found that TGFβ treatment significantly increased the ARK5 expression level. Additionally, ARK5 silencing led to a decreased fibrotic response of the renal epithelial cells to TGFβ1, indicating that ARK5 is a necessary, intrinsic component for the completion of the TGFβ signalling pathway. Furthermore, Suzuki et al. studied the mechanism of survival of human hepatoma cells in hypoxic environments [57]. It is already established that ARK5 is involved with austerity of cancer cells. However, this study demonstrated that ARK5 was directly activated by TGFβ, and that TGFβ inhibition attenuated the action of ARK5. Thus, Suzuki et al. concluded that the role of ARK5 in cancer cell austerity is activated and upregulated by TGFβ.
AMPK Inhibition of Mtor In Ocular Diseases and Glaucoma
Belforte et al. demonstrated that AMPK was upregulated and mTOR was suppressed in the RGC of mice with ocular hypertension, when compared with the control group [61] [Figure 2]. Inhibiting AMPK using siRNA or compound C resulted in a restoration of mTOR signaling and downstream enhanced axonal survival. Finally, Belforte et al. studied RGCs of a small group of patients with glaucoma (n=10) and found that AMPK was upregulated in the RGC cells of this patient population versus age matched controls [61]. Interestingly, mTOR is a downstream pathway which is negatively regulated by AMPK [62]. However, whilst AMPK activation primarily occurs in states of cellular distress, mTOR is activated in reverse conditions, and requires positive signals and nutrients availability from growth factors and nutrients [62]. Furthermore, mTORC1 becomes inactivated by cellular stresses such as glucose deficiency, or hypoxia, thereby preventing cellular growth and differentiation by inducing cell cycle arrest. AMPK has been shown to suppress and downregulate mTOR, through direct phosphorylation of TSC1/2 tumor suppressor genes, and also its scaffold protein, raptor. Thus, AMPK exerts its inhibition of mTOR through both direct and indirect mechanisms [63,64]. This dynamic relationship demonstrates that AMPK acts as a metabolic check point of cellular energy status.
Metformin as A Treatment For Glaucoma
Metformin is a biguanide therapeutic drug probably best known for its hypoglycaemic properties in the treatment of T2DM. The mechanism of action of metformin is still unclear, although several mechanisms of its action have been proposed, such as its inhibition of complex-1 mitochondrial chain and its activation of AMPK. Although it is well known that metformin possesses antifibrotic and anti-tumorigenic properties, its role as an antifibrotic therapeutic in the eye is largely unknown. Lin et al conducted a study of 150,061 patients diagnosed with diabetes mellitus (DM), and found that 5893 (3.9%) patients were also diagnosed with open angle glaucoma (OAG) [65]. Interestingly, they found that there was a 25% relative risk reduction associated with the development of glaucoma in patients taking high doses of metformin (>1100g over 2 years) versus patients not on metformin, including patients who were prescribed other oral hypoglycaemic medication. This study demonstrates a potential neuroprotective role for metformin in the development of glaucoma and highlights its potential as a future therapeutic target for glaucoma therapy. The results of a recent study demonstrated that metformin delivered as eye drops eliminated post-operative fibrosis and inflammation in rat models post glaucoma filtration surgery (GFS) via direct activation of the AMPK/Nrf2 signalling pathway [66]. Li et al. found that human conjunctival fibroblasts (HConF) pre-treated with metformin demonstrated dramatically decreased levels of fibrosis and oxidative stress when compared to control group (without GFS), GFS group, and rats treated with mitomycin C (MMC). Metformin proved to be just as effective at decreasing TGF- β2 driven fibrosis and proliferation of HConF cells as the established AMPK activator 5-aminoamidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR). Furthermore, inhibition of both nuclear factor erythroid 2-related factor 2 (Nrf2) and AMPK diminished the anti-fibrotic effect of metformin. Analysis of immunohistochemical staining of post- operative conjunctival tissue revealed decreased AMPK levels and upregulated aSMA expression in damaged tissue when compared to the normal control group [66]. The reduction in AMPK in diseased tissue is thought to be as a result of inflammatory markers (platelet derived growth factor (PDGF) and TGFB) inhibiting AMPK expression [67]. What remains to be elucidated is whether metformin has any therapeutic effect in reversing already established fibrosis in HConF cells. This study highlights the antifibrotic mechanism of metformin in fibrotic eye disease through the activation of the AMPK/Nrf2 signalling pathway.
Future Directions and Conclusion
The cellular mechanisms involved in glaucoma are yet to be fully elucidated. Understanding these processes will lead to new methods of preventing chronic glaucomatous vision loss when conventional IOP-lowering treatments either fail to prevent progression (up to 20% of glaucoma patients continue to progress to blindness) or adequate target pressures cannot be achieved due to ineffectiveness or poor tolerance to medication. Thus, there is a clear unmet need to target the underlying mechanisms governing the progressive fibrotic remodelling of the LC, to halt the progressive and ongoing fibrotic damage to the LC/ONH and visual field loss. It is clear that there are multiple systemic cellular processes at play simultaneously, and when combined, result in the development of glaucoma. Glaucoma may be described as a multifactorial disease entity, and it is likely that in the future, there will be no ‘one size fits all’ therapeutic option. It is evident, however, that mitochondrial dysfunction plays an integral role in the aetiology of glaucoma, and that the dysregulation of this organelle directly results in RGC susceptibility and vulnerability. Several studies have highlighted promising therapeutic targets in halting or even reversing this progression, including nicotinamide, insulin and metformin. AMPK is a highly conserved master regulator of metabolism, both at the cellular and organismal levels, whose function is extremely relevant not only for normal physiology, but also for the understanding of many metabolic diseases [54]. The examination of novel mechanisms of AMPK as the key metabolic sensor in LC cells in glaucoma will be vital to understand the driving force underlying fibrotic changes occurring in the LC. These activated LC myofibroblasts drive the fibrotic processes occurring in the LC. The glaucoma LC cells adapt to their pro-fibrotic role by increasing their proliferation, reducing apoptosis, and augmenting their metabolism. These activated LC myofibroblasts essentially undergo ‘metabolic reprogramming’ to utilise alternative high-energy sources to enhance cellular growth and development. Halting the pro-fibrotic activity and metabolism of glaucoma LC cells by restoring AMPK expression and activity to normal levels, could lead to a new therapeutic approach to reduce fibrosis in glaucoma. In conclusion, glaucoma is a multifactorial, progressive, chronic optic neuropathy. The second leading cause of irreversible blindness worldwide, the development of novel therapeutics to combat this disease is of paramount importance. This review has identified several key metabolic sensors whose dysregulation and dysfunction directly drive and promote disease development and progression. Whilst there have been several potential treatment options investigated for this disease in the last decade, it is clear that further research and clinical trials to fully determine the suitability and effectiveness of therapeutic targets are necessary.
Conflict of Interest
There is no economic interest or conflict of interest
References
- Quigley H, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90(3): 262-267.
- Crawley L, Zamir SM, Cordeiro MF, Guo L (2012) Clinical Options for the Reduction of Elevated Intraocular Pressure. Ophthalmol Eye Dis 4: 43-64.
- McDonnell F, O Brien C, Wallace D (2014) The role of epigenetics in the fibrotic processes associated with glaucoma. J Ophthalmol 2014(7): 750459.
- Wallace DM, Murphy Ullrich JE, Downs JC, O Brien CJ (2014) The role of matricellular proteins in glaucoma. Matrix Biol 37: 174-182.
- McElnea EM (2011) Oxidative stress, mitochondrial dysfunction, and calcium overload in human lamina cribrosa cells from glaucoma donors. Mol Vis 17: 1182-1191.
- Yang H (2011) Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Investig. Ophthalmol Vis Sci 52(10): 7109-7121.
- Tezel G, Trinkaus K, Wax MB (2004) Alterations in the morphology of lamina cribrosa pores in glaucomatous eyes. Br J Ophthalmol 88(2): 251-256.
- White E, Mantovani A (2014) Spectrum of Tissue Injury and Resolution. J Pathol 229(2): 141-144.
- Diegelmann RF, Melissa C, Evans (2014) Wound Healing: An Overview of Acute, Fibrotic and Delayed Healing. Front Biosci 9: 283-289.
- Betensley A, Sharif R, Karamichos DA (2016) Systematic Review of the Role of Dysfunctional Wound Healing in the Pathogenesis and Treatment of Idiopathic Pulmonary Fibrosis. J Clin Med 6(1): 2.
- Kirwan RP, Wordinger RJ, Clark AF, O Brien CJ (2009) Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Mol Vis 15: 76-88.
- Kirwan RP, Felice L, Clark AF, O Brien CJ, Leonard MO (2012) Hypoxia regulated gene transcription in human optic nerve lamina cribrosa cells in culture. Invest Ophthalmol Vis Sci 53(4): 2243-2255.
- Kirwan RP, Leonard MO, Murphy M, Clark AF, OBrien CJ (2005) Transforming Growth factor-β-regulated gene transcription and protein expression in human GFAP-negative lamina cribrosa cells. Glia 52(4): 309-324.
- Papageorgis P (2015) TGF β signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol 2015: 587183.
- Liu B (2018) Increased substrate stiffness elicits a myofibroblastic phenotype in human lamina cribrosa cells. Investig. Ophthalmol Vis Sci 59(2): 803-814.
- Zode GS (2011) Transforming growth factor-β2 increases extracellular matrix proteins in optic nerve head cells via activation of the smad signaling pathway. Mol Vis 17: 1745-1758.
- Kolliopoulos C (2019) Transforming growth factor (TGF) induces NUAK kinase expression to fine-tune its signaling output. J Biol Chem 294(11): 4119-4136.
- Schmitt, Segert (2008) 基因的改变 NIH Public Access. Bone 23: 1-7.
- Inman DM, Harun Or Rashid M (2017) Metabolic vulnerability in the neurodegenerative disease glaucoma. Front Neurosci 11: 1-19.
- He Y, Ge J, Tombran Tink J (2008) Mitochondrial defects and dysfunction in calcium regulation in glaucomatous trabecular meshwork cells. Investig Ophthalmol Vis Sci 49(11): 4912-4922.
- Lascaratos G (2015) Resistance to the most common optic neuropathy is associated with systemic mitochondrial efficiency. Neurobiol Dis 82: 78-85.
- Coughlin L, Morrison RS, Horner PJ, Inman DM (2015) Mitochondrial morphology differences and mitophagy deficit in murine glaucomatous optic nerve. Investig Ophthalmol Vis Sci 56(3): 1437-1446.
- Ju W (2009) NIH Public Access. 49: 4903-4911.
- Williams PA, Harder JM, John S WM (2017) Glaucoma as a Metabolic Optic Neuropathy: Making the Case for Nicotinamide Treatment in Glaucoma. J Glaucoma 26(12): 1161-1168.
- Kamel K, Farrell M, O Brien C (2017) Mitochondrial dysfunction in ocular disease: Focus on glaucoma. Mitochondrion 35: 44-53.
- Harun Or Rashid M (2020) MCT2 overexpression rescues metabolic vulnerability and protects retinal ganglion cells in two models of glaucoma. Neurobiol Dis 141: 104944.
- Lee S (2012) Impaired complex-I-Linked respiration and ATP synthesis in primary open-angle glaucoma patient lymphoblasts. Investig Ophthalmol Vis Sci 53(4): 2431-2437.
- Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT (2012) Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta Bioenerg 1817(6): 851-862.
- Van Bergen NJ (2015) Measurement of systemic mitochondrial function in advanced Primary Open-Angle Glaucoma and leber hereditary optic neuropathy. PLoS One 10(10): 1-18.
- Khawaja AP (2016) Assessing the association of mitochondrial genetic variation with primary open-angle glaucoma using gene-set analyses. Investig Ophthalmol Vis Sci 57(11): 5046-5052.
- Kamel K (2020) Reduced Oxidative Phosphorylation and Increased Glycolysis in Human Glaucoma Lamina Cribrosa Cells. Invest Ophthalmol Vis Sci 61(13): 4.
- Shirlaw JT (1931) The metabolism of tumours. Br Med J 1: 74.
- Pavlides S (2012) Warburg meets autophagy: Cancer-Associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxidants Redox Signal 16(11): 1264-1284.
- Mitchell SJ (2019) HHS Public Access mice. 27: 667-676.
- Hui F (2020) Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin Exp Ophthalmol 48(7): 903-914.
- Katsyuba E, Auwerx J (2017) Modulating NAD + metabolism, from bench to bedside. EMBO J 36(18): 2670-2683.
- Aman Y, Qiu Y, Tao J, Fang EF (2018) Therapeutic potential of boosting NAD+ in aging and age-related diseases. Transl Med Aging 2: 30-37.
- Cantó C (2012) The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15(6): 838-847.
- Gilley J, Coleman MP (2010) Endogenous Nmnat2 Is an Essential Survival Factor for Maintenance of Healthy Axons. PLoS Biol 8(1): e1000300.
- Hwang ES, Song SB (2020) Possible adverse effects of high-dose nicotinamide: Mechanisms and safety assessment. Biomolecules 10(5): 1-21.
- Harrison IF, Powell NM, Dexter DT (2019) The histone deacetylase inhibitor nicotinamide exacerbates neurodegeneration in the lactacystin rat model of Parkinson’s disease. J Neurochem 148(1): 136-156.
- Fukushima T (2005) Niacin metabolism and Parkinson’s disease. Environ. Health Prev Med 10(1): 3-8.
- Ulanovskaya AO, Zuhl MA, Cravatt FB (2016) NNMT promotes epigenetic remodeling in cancer by creating a methylation sink. 97: 5421-5433.
- Akintunde A (2017) Physiological Phenotyping for Personalized Therapy of Uncontrolled Hypertension in Africa. Am J Hypertens 30(9): 923-930.
- Hemati T, Moghadami Tabrizi N, Davari Tanha F, Salmanian B, Javadian P (2011) High plasma homocysteine and insulin resistance in patients with polycystic ovarian syndrome. Iran J Reprod Med 9(3): 223-228.
- Liu M (2017) Serum N1-methylnicotinamide is associated with coronary artery disease in Chinese patients. J Am Heart Assoc 6(2).
- Kannt A (2018) A small molecule inhibitor of Nicotinamide N-methyltransferase for the treatment of metabolic disorders. Sci Rep 8(1): 3660.
- Emerman AB, Zhang ZR, Chakrabarti O, Hegde RS (2010) Trehalose Is a Key Determinant of the Quiescent Metabolic State That Fuels Cell Cycle Progression upon Return to Growth. Mol Biol Cell 21: 4325-4337.
- Lin SC, Hardie DG (2018) AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab 27(2): 299-313.
- Chatterjee A, Villarreal G, Oh DJ, Kang MH, Rhee DJ (2014) AMP-activated protein kinase regulates intraocular pressure, extracellular matrix, and cytoskeleton in trabecular meshwork. Investig. Ophthalmol Vis Sci 55(5): 3127-3139.
- Harun Or Rashid M, Inman DM (2018) Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J Neuroinflammation 15(1): 1-15.
- Zhang CS (2014) The lysosomal v-ATPase-ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab 20(3): 526-540.
- Claus SP (2019) The Strange Case of Prevotella copri: Dr. Jekyll or Mr. Hyde? Cell Host Microbe 26(5): 577-578.
- Bambang A, Tanadi C, Sumarpo A (2019) Deciphering the role of AMPK-related kinase 5 in human cancer progression and metastasis. Biomed Res Ther 6(10): 3396-3404.
- Huang X, Lv W, Zhang JH, Lu DL (2014) MiR-96 functions as a tumor suppressor gene by targeting NUAK1 in pancreatic cancer. Int J Mol Med 34(6): 1599-1605.
- Chen D (2017) Knockdown of ARK5 Expression Suppresses Invasion and Metastasis of Gastric Cancer. Cell Physiol Biochem 42(3): 1025-1036.
- Suzuki A (2005) Involvement of transforming growth factor-β1 signaling in hypoxia-induced tolerance to glucose starvation. J Biol Chem 280(36): 31557-31563.
- Zhang HY, Li JH, Li G, Wang SR (2015) Activation of ARK5/miR-1181/HOXA10 axis promotes epithelial-mesenchymal transition in ovarian cancer. Oncol Rep 34(3): 1193-1202.
- Bell RE (2014) Transcription factor/microRNA axis blocks melanoma invasion program by miR-211 targeting NUAK1. J Invest Dermatol 134(2): 441-451.
- Brennan EP (2012) Next-generation sequencing identifies TGF-β1-associated gene expression profiles in renal epithelial cells reiterated in human diabetic nephropathy. Biochim Biophys Acta Mol Basis Dis 1822(2): 589-599.
- Belforte N, Cueva Vargas JL, Di Polo A (2018) AB015 Metabolic stress in glaucoma engages early activation of the energy biosensor adenosine monophosphate-activated protein kinase leading to neuronal dysfunction. Ann Eye Sci 3: AB015-AB015.
- Reuben J Shaw (2009) LKB1 and AMPK control of mTOR signalling and growth Reuben. Acta Physiol (Oxf) 196(1): 65-80 (2009).
- Cork GK, Thompson J, Slawson C (2018) Real talk: The inter-play between the mTOR, AMPK, and hexosamine biosynthetic pathways in cell signaling. Front Endocrinol (Lausanne) 9: 1-9.
- Gwinn DM (2008) AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol Cell 30(2): 214-226.
- Ogretmen B (2019) 乳鼠心肌提取 HHS Public Access. Physiol Behav 176: 139-148.
- Li X (2020) Eye Drops of Metformin Prevents Fibrosis After Glaucoma Filtration Surgery in Rats via Activating AMPK/Nrf2 Signaling Pathway. Front Pharmacol 11: 1-14.
- Bone NB (2018) Metformin reverses established lung fibrosis in a bleomycin model. Na Med 24(8): 1627-1627.