Lupine Publishers Group
Lupine Publishers
Review ArticleOpen Access
A Review of Achromatopsia Volume 3 - Issue 1
Sandra Abeijón Martínez*
- School of Advanced Education, Research and Accreditation (SAERA)
Received:September 02, 2020; Published: September 21, 2020
Corresponding author: Sandra Abeijón Martínez, School of Advanced Education, Research and Accreditation (SAERA)
DOI: 10.32474/TOOAJ.2020.03.000154
Abstract
Congenital achromatopsia is a hereditary form of day blindness caused by cone photoreceptor dysfunction, with an incidence of approximately 1 in 30,000. This inherited disorder is characterized by a lack of color discrimination, nystagmus, photophobia, and low visual acuity (< 0.2). The most typical genetic mutations are autosomal recessive changes in CNGA3, CNGB3, GNAT2, PDE6H, PDE6C, or ATF6. It should not be confused with cerebral achromatopsia, which is an acquired form of total color blindness that can result from illness, trauma, or some other cause. Color plays an essential role in our lives. It can change actions, influence thinking, and cause reactions. As a powerful type of communication, color is irreplaceable. Green means “go” and red means “stop”. Traffic lights send this global meaning. Color vision deficiency, creates challenges in the daily lives of those who have an insufficient visual sense.
Purpose: The aim of this review is to examine the literature published on achromatopsia and summarize the diagnosis, management, genetic characteristics, and the recent advances in gene therapy.
Conclusions: Congenital achromatopsia is a complex inherited disease. Management of achromatopsia is multifaceted. There is currently no cure for achromatopsia, although gene therapy is a therapeutic option already being studied in clinical trials. The most recent study in human adults can be classified as safe and positive in terms of efficacy.
Keywords: Achromatopsia (ACHM); Gene therapy
Introduction
Achromatopsia is a condition characterized by a partial or total
absence of color vision [1]. People with complete achromatopsia
cannot perceive any colors; they only see white, black, and shades of
gray. Incomplete achromatopsia is a milder form of the disease that
allows some color discrimination [1]. Oliver Sacks, in The Case of
Color-blind Painter [2], transcribe the disturbing story of one of his
cerebral achromatopsia patients after a car accident in the following
way: Mr. I. could hardly tolerate the changed aspects of people (“like
animated grey statues”) any more than he could tolerate his own
appearance in the mirror: he shunned social intercourse and found
sexual intercourse impossible. He saw people’s fesh, his wife’s fesh,
his own fesh, as an abhorrent grey; “fesh-colored” now appeared
“rat-colored” to him. Congenital achromatopsia also involves other
problems with vision, including an increased sensitivity to light and
glare (photophobia), involuntary back-and-forth eye movements
(nystagmus), significantly reduced sharpness of vision (low visual
acuity) [1], usually lower than 20/200 because of central vision
loss, and reduced conemediated electroretinographic (ERG)
amplitudes [3]. Affected individuals can also have vision problems
in the first few months of life, such as hyperopia (farsightedness) or,
less commonly, myopia (nearsightedness).
Achromatopsia is different from the more typical forms of color
vision deficiency (also called color blindness), in which people
can perceive color but have difficulty distinguishing between
specific colors, such as red and green [1]. In this disease, cone
photoreceptors, the retinal sensory neurons mediating color vision,
seem viable but fail to generate an electrical response to light [4,5].
All individuals with achromatopsia (achromats) have impaired color
discrimination along all three axes of color vision corresponding to
the three cone classes [6]. Cerebral achromatopsia is an acquired
disorder caused by injury to the cerebral cortex of the brain, instead
of defectiveness in the cells of the eye’s retina. This condition is often mistaken for congenital achromatopsia but physiological deficits
of both disorders are completely different. It is a consequence of
cortical damage that arises through ischemia or infarction of a
specific area in the ventral occipitotemporal cortex of humans [7].
This damage is almost always the result of injury or illness [8].
Medical history and age at presentation will help to differentiate
this condition from congenital achromatopsia. Additionally, these
patients nearly always have additional neurologic symptoms
beyond the loss of color vision [8].
Epidemiology
Achromatopsia is a relatively uncommon disorder that affects an estimated 1 in 30,000 people worldwide [9]. Complete achromatopsia is more common than incomplete achromatopsia. Its incidence varies in different parts of the world. Because there is a genetic link, it is more common in regions where there is a high rate of consanguineous marriages (marriages between relatives) and in the eastern Pacific islands of Pingelap [10], approximately five percent of the atoll’s 3,000 inhabitants are afflicted [11,12]. The people of this region have termed achromatopsia “maskun”, which literally means “not see” in Pingelapese [13]. The condition can be traced back to 1775, when Typhoon Lengkieki decimated the population in the island, leaving only a handful of survivors to repopulate the islands. One of the few survivors was a carrier of the rare genetic mutation and passing it on to his descendants, he became the genetic founder for the vision disorder four generations later [14]. Oliver Sacks wrote his book, The Island of the Colorblind (1997), which story chronicles Oliver Sack’s 1994 quest with Knut Nordby and Bob Wasserman to the isolated atoll of Pingelap in Micronesia.
Photoreceptors: rods and cones
Figure 1: Schematic diagram of vertebrate rod and cone photoreceptors. Retrieved from Photoreceptor Phosphodiesterase (PDE6): A G- Protein-Activated PDE Regulating Visual Excitation in Rod and Cone Photoreceptor, Cells Rick H Cote 2006.
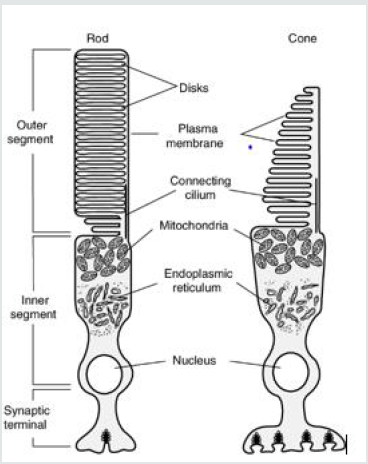
Rod and cone photoreceptors are specialized neurons that
function in the initial step of vision [15]. These light-sensitive cells
lie at the back of the retina adjacent to the retinal pigment epithelium
(RPE), a cell layer that is vital for the survival of photoreceptors
[15]. Cones are mostly in the center of the retina. When retinitis
pigmentosa affects them, there is a slowly loss of central vision
and of the ability to see colors [15]. Rods work at very low levels
of light. Only a few bits of light (photons) can activate a rod, that’s
why we use these for night vision. Rods don’t help with color vision,
which is why at night, we see everything in a gray scale [16]. Rods
are more numerous, about 120 million, and more sensitive than
cones. However, they are not sensitive to color. Rods are much more
concentrated in the macula (central yellow spot) and they provide
the eye’s color sensitivity. In the center of the macula is the “fovea
centralis”, a 0.3 mm diameter rod-free area with densely packed
and very thin cones. Rod and cone photoreceptors have similar
structures (Figure 1). Photoreceptors have diameters ranging from
1 to 4 μm, being smaller at the fovea, enhancing visual acuity there.
The inner segment contains the nucleus and has an axon-like process
connected to a synaptic terminal. The outer segment in the cone cell
has its plasma membrane invaginated into numerous closely packed
parallel folds [17], forming discs. In rod cells the discs are pinched
off the plasma membrane to become completely intracellular [17].
The disc membrane is densely packed with visual pigment. In rod
cells, this is rhodopsin. Each type of cone cells has its characteristic
cone opsin. The outer segment is continually regenerated from the
base, whilst its apical tip is phagocytosed by pigment epithelial cells
at the rate of 3-4 discs per hour. Photoreceptors are incapable of
mitotic division [17].
In 1965 came experimental confirmation of a long-expected
result - there are three types of color-sensitive cones in the retina
of the human eye, red or L cones (long wavelength-64%), green or
M cones (medium wavelength-32%), and blue or S cones (short
wavelength-2%). They are each sensitive to different portions of
the spectrum of light. Visible colors correspond to light wavelengths
ranging from 400 to 700 nm [16]. Even though each cone is most
sensitive to a specific color of light (where the line peaks), they also
can detect other colors (Figure 2) [16].
Figure 2: The three types of cones, capable of recognizing light wavelengths that peak at green, red and blue. Retrieved from Rods and Cones, C J Kazilek, Kim Cooper 2010.
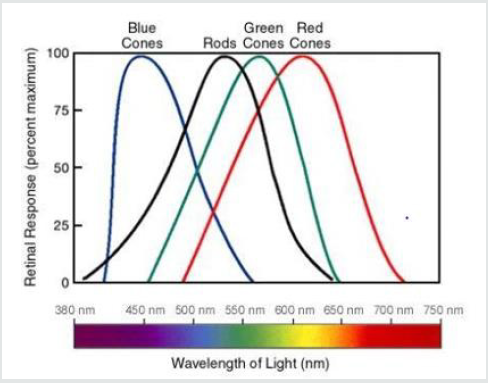
The “blue” cones are sensitive to the peak of the light response
curve at about 445nm. They are found outside the fovea centralis
where the green and red cones are concentrated and they
constitute only about 2% of the total number of cones. Their
disadvantage in number is clear but, on the contrary, they are much
more light sensitive than green and red cones [18]. However, the
blue sensitivity of the final visual perception is equivalent to that
of red and green, proposing that there is a somewhat selective
“blue amplifier” somewhere in the visual processing in the brain
[18]. Morphological studies have allowed us to differentiate the
short wavelength specific (blue) cone from the medium and long
wavelength specific cones in the human retina even without special
antibody staining techniques [19]. S-cones have longer inner
segments that project further into subretinal space than longer
wavelength cones (Figure 3).
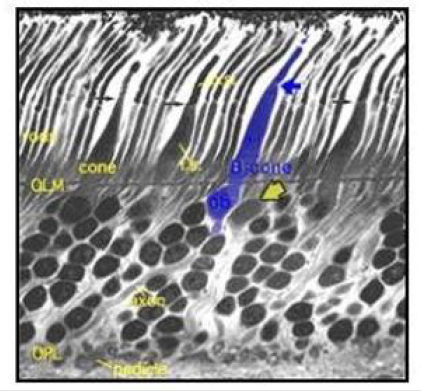
The S-cones also have smaller and morphologically different
pedicles than the other two wavelength cones. Throughout the
retina, the S-cones have a different distribution and do not fit into
the regular hexagonal mosaic of cones typical of the other two types
[20].
Figure 3: Vertical section of Human S-cone. Retrieved from The Organization of the Retina and Visual System, Kolb 1995.
Different kinds of color blindness
Most of us have the ability to perceive a million different colors
[21]. This is due to the presence of the three types of cones in our
eyes that send color messages to our brain. Some people who have
a faulty cone or only two cones can suffer a greatly decreased color
perception. This condition is known as color blindness [21].
That color vision is trichromatic was first recognized by Thomas
Young at the beginning of the nineteenth century (thus, people
with normal color vision are called trichromats). Trichromats
have normal cones and light sensitive pigment and they can see all
the different colors and delicate mixtures of them by using cones
sensitive to one of three light wavelengths (red, green, and blue).
Monochromacy and Achromatopsia, also known as “total color
blindness” [22], is the lack of ability to distinguish colors; caused
by cone defect or absence [23]. Monochromacy occurs when two or
all three of the cone pigments are missing and color and lightness
vision is reduced to one dimension [22].
Cases of achromatopsia are classified as complete when the
patient has a total loss of color discrimination or incomplete
when this loss of color discrimination is partial. The variability
across this spectrum is believed to be due, in part, to the degree
of residual cone photoreceptor function. Patients with complete
achromatopsia have a total absence of color discrimination, and
they are only able to perceive white, black, and shades of gray.
Complete achromatopsia is caused by the total loss of function of
all three types of cone photoreceptors (long-wavelength, mediumwavelength,
and short-wavelength) [24] (Figure 4).
Figure 4: Simulation of daylight vision in Achromatopsia patients. Retrieved from Gene therapy for CNGA3-linked achromatopsia: from mouse models to clinical trials, Stylianos 2017.
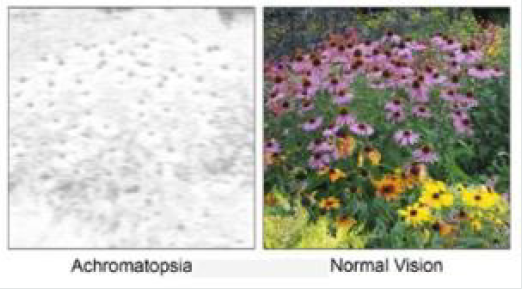
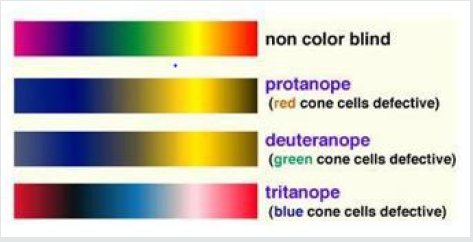
Patients with incomplete achromatopsia have better color discrimination and visual acuity, in comparison to patients with complete achromatopsia. Some patients with incomplete achromatopsia have particular types of color vision impairment, such as a loss of photoreceptor function in medium-wavelength cones but residual function in short-wavelength and long-wavelength cones [24,25]. This example of incomplete achromatopsia is associated with deuteranopia-like green color blindness, but it is distinct from true X-linked deuteranopia’s, which are caused by mutations in the gene that encodes medium-wavelength-sensitive opsin [26]. Achromatopsia and Monochromacy describes a range of conditions that include Achromatopsia, rod-Monochromacy and S-cone Monochromacy. Sometimes, these are collectively referred to as types of achromatopsia, as the word “achromat” meaning “no color” [27]. However, not all cases of achromatopsia have “no color” vision. Trichromats, Dichromats and Monochromats are terms used in the vision science community to refer to different possible configurations of the human visual system having three (tri-), di (two) or one (mono) channel of color information [27]. People with dichromatic color vision have only two types of cones which are able to perceive color, that is, they have a total absence of function of one cone type [28]. Lack of ability to see color is the simplest way to explain this condition but in actual fact it is a specific section of the light spectrum which can’t be perceived. For convenience we call these areas of the light spectrum ‘blue’, ‘green’ or ‘red’. The sections of the light spectrum which the ‘green’ and ‘red’ cones perceive overlap and this is why green and red color vision deficiencies are often known as red/green color blindness and why people with red and green deficiencies see the world in a similar way [28]. Dichromats exist in three different types according to the missing cone: protanopia (missing L-cone red), deuteranopia (missing M-cone green), and tritanopia (missing S-cone blue) (Figures 5 & 6).
Figure 5: Simulation of colorblind vision. Retrieved from Color Universal Design (CUD) How to make figures and presentations that are friendly to Colorblind people, Okabe and Ito 2008.
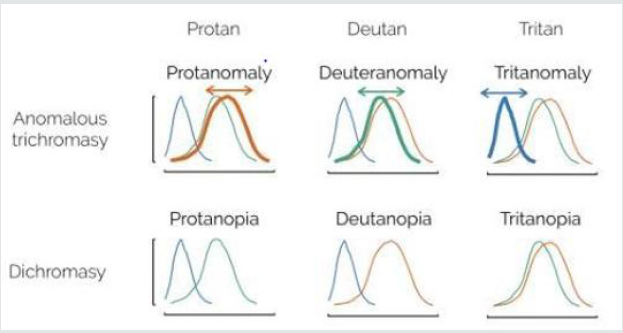
People with ‘defective’ trichromatic vision will be color blind to some degree and are known as anomalous trichromats. In people with this disorder all of their three cone types are used to perceive light colors but one type of cone perceives light slightly out of alignment, so that there are three different types of effect produced depending upon which cone type is ‘defective’ [28]. The different anomalous conditions are protanomaly, which is a diminished sensitivity to red light, deuteranomaly which is a diminished sensitivity to green light and is the most usual form of color blindness and tritanomaly which is a diminished sensitivity to blue light and is extremely rare [28]. About half of people with anomalous trichromacy will see the world in a similar way to those with dichromacy but their capacity to perceive colors will worsen in poor light and improve in good light. Often their color perception can be as poor as it is for those with dichromacy [28] (Figure 7).
Figure 7: Color vision deficiencies can be either due to a mutation that shifts one of the color cones or a lack of one of them. The affected color cone can be red (protan), green (deutan) or blue (tritan). Figured adapted from Okatobe and Ito 2008.
Tetrachromatic color vision allows an individual to discriminate colors along a perceptual dimension that is unavailable to the normal person. Most people’s color perception is “trichromatic” [29]. Organisms with tetrachromacy are called tetrachromats. For these organisms, the perceptual effect of any arbitrarily chosen light from its visible spectrum can be matched by a mixture of no fewer than four different pure spectral lights [30]. The normal explanation of tetrachromacy is that the organism’s retina contains four types of cone cells with different absorption spectra [30]. This means the animal may see wavelengths beyond those of a typical human being’s eyesight, and may be able to differentiate colors that to a human are identical. However, the relationship between the existence of a fourth class of retinal cone and the dimensionality of color vision is more complex than previously thought [29]. The zebrafish (Danio rerio) is an example of a tetrachromat, containing cone cells sensitive for red, green, blue, and ultraviolet light [31]. Tetrachromacy is expected to occur in several species of birds, fish, amphibians, reptiles, arachnids, and insects.
Phototransduction
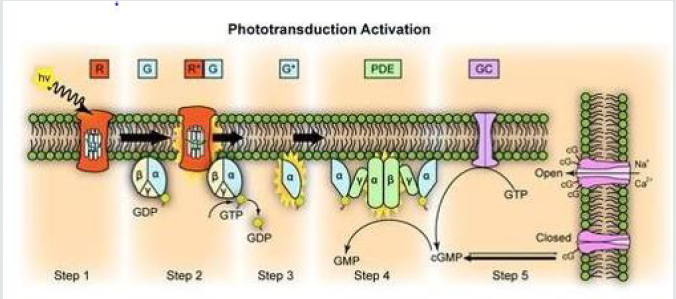
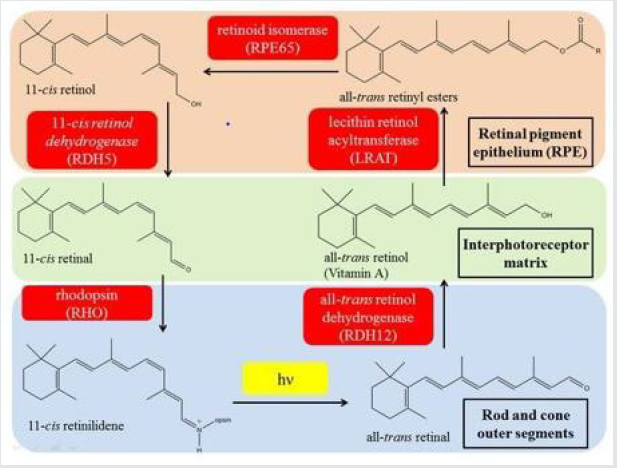
Phototransduction is a process by which light is converted into electrical signals in the rod cells, cone cells and photosensitive ganglion cells of the retina of the eye. The visual cycle is the biological transformation of photons into an electrical signal in the retina. This process occurs through G-protein coupled receptors called opsins which contain the chromophore 11-cis retinal. 11- cis retinal is the chromophore present in all photoreceptors; it is a derivative of vitamin A [32]. Phototransduction in rods is initiated when light isomerizes the 11-cis-retinal chromophore of rhodopsin to its all-trans isomer that induces a conformational change [33- 36]. 11-cis retinal is covalently linked to the opsin receptor via Schiff base forming retinylidene protein. Opsin therefore undergoes a conformational change to metarhodopsin II, which activates the regulatory G protein transducin (Gt). The alpha subunit of Gt activates phosphodiesterase, also known as PDE6, which hydrolyses cGMP to 5’-GMP. As high level of cGMP keeps cGMP-gated sodium channels open, the lowering of cGMP levels closes these channels [37] which causes hyperpolarization of the cell due to the ongoing efflux of potassium ions. Subsequently, hyperpolarization of the cell causes closure of voltage-gated calcium channels. As calcium levels in the photoreceptor cell drop, the level of the neurotransmitter glutamate also drops. This is because calcium is necessary for the glutamate-containing vesicles to fuse with the cell membrane and liberate their contents. A decrease in the amount of glutamate liberated by the photoreceptors causes hyperpolarization of cone off-center bipolar cells and depolarization of on-center bipolar cells (rod and cone On bipolar cells). This effectively relays the light signal to postsynaptic neurons as an electrical signal [38-43] (Figures 8 & 9).
Figure 9: The visual phototransduction cycle yields 11-cis-retinal from 11-cis-retinol using 11-cis-retinol dehydrogenase. Retrieved from the 11-cis-retinol dehydrogenase activity of RDH10 and its interaction with visual cycle proteins, Thompson [42,43].
Causes
Achromatopsia can be acquired or congenital. Brain damage
can produce acquired deficiencies of color perception, or cerebral
achromatopsia. Cerebral achromatopsia is a type of acquired color
blindness that is caused by damage to the cerebral cortex of the
brain, rather than defectiveness in the cells of the eye’s retina. In
patients with cerebral achromatopsia, lesions tend to overlap on
a restricted region in the ventral occipitotemporal cortex, close
to the reported locations of the putative V4 complex and to foci of
increased blood-oxygen-level- dependent (BOLD) activity related to
color perception in normal participants [44]. As an only sequel to a stroke, cerebral achromatopsia is rare [45]. The common causes of
the congenital forms of achromatopsia are all caused by malfunction
of the retinal phototransduction pathway. Specially, this form of
achromatopsia seems to result from the inability of cone cells to
properly respond to light input by hyperpolarizing. Achromatopsia
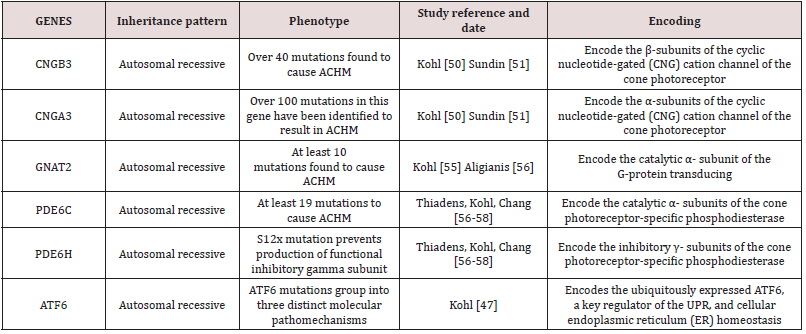
is an autosomal recessive disease that is caused by mutations in any
of six associated genes that have been identified to date: CNGA3,
CNGB3, GNAT2, PDE6H, and ATF6 [46,47]. Five of the genes that
have been associated to achromatopsia encode proteins that impact
phototransduction in cone photoreceptors. Approximately half of
achromatopsia cases are caused by mutations in the CNGB3 gene
[48], and about a quarter are caused by mutations in the CNGA3
gene [49]. Mutations in the genes CNGA3 and CNGB3 encoding the
α and β subunits of cone photoreceptor CNG channels have been
described in subjects with achromatopsia [49-52]. CNG channels in
the cone photoreceptor are composed of three α subunits and one
β subunit, and are located in the plasma membrane of the outer
segment [53,54]. When open, CNG channels allow the nonselective
flow of cations across the photoreceptor membrane. These proteins
are functionally important in all three classes of human cones.
GNAT2, mapped to locus ACHM4 on chromosome 1p13,
encode the catalytic α-subunit of the G-protein transducing
[55,56] and PDE6C (ACHM5) and PDE6H encode the catalytic
α- and the inhibitory γ-subunits, respectively, of the cone
photoreceptor-specific phosphodiesterase [55,57-58]. Recently,
ATF6 was identified as a new ACHM gene, suggesting an essential
and unpredicted role of ATF6A in human foveal development
and cone function. This was surprising as ATF6 encodes the
ubiquitously expressed ATF6, a key regulator of the UPR, and
cellular endoplasmic reticulum (ER) homeostasis. This adds to the
list of genes that, despite a ubiquitous expression, when mutated
can result in an isolated retinal photoreceptor phenotype [47].
Mutations in GNAT2 and PDE6C are rare and are found in fewer
than 2% of all achromats [59]. ACHM is transmitted in an autosomal
recessive manner, which means that for the disease to develop, the
two copies or alleles of the gene should be mutated. Normally, each
gene in the body has two copies or alleles, one allele comes from
the mother and the other allele comes from the father. A carrier is
a person who only has one copy or allele of the mutated gene and
does not have achromatopsia. For achromatopsia to develop, an
individual must inherit one allele of the mutated gene from each
parent. This means that both parents must be either carriers or have
the disorder involving mutations in the same achromatopsia gene.
If both parents are carriers, each sibling of an affected individual
with achromatopsia has a 25% chance of having the disease, a 50%
chance of being an asymptomatic carrier, and a 25% chance of not
having the disease and not being a carrier [10].
Symptoms
The clinical signs and symptoms of achromatopsia vary.
Achromatopsia is characterized by a pendular nystagmus,
reduced visual acuity, a small central scotoma, eccentric fixation,
an increased sensitivity to light (photophobia) and reduced or
complete loss of color discrimination. All achromats have impaired
color discrimination along all three color vision axes, each one
corresponding to the three cone classes. These symptoms usually
start at birth or early infancy and their severity varies depending on
genetic and phenotypic variability. Visual acuity is usually less than
20/200 for those with complete achromatopsia but may be as good
as 20/80 for those with incomplete achromatopsia. Visual acuity is
usually stable [60], in contrast to other cone dystrophies in which
visual acuity progressively worsens.
Clinically, the first signs of achromatopsia in infants are the
presence of nystagmus, a pendular quivering of the eyes, and
photophobia as evidenced by squinting in bright light [60]. In usual
complete achromatopsia, patients present typically by 6 months
old with nystagmus and photophobia. As one example, many
achromats have been given the diagnosis of “congenital nystagmus.”
Nystagmus (involuntary movement of the eyes) is a symptom of
achromatopsia, one that is especially obvious during infancy and
childhood, but having this symptom is not the same as having the
medical eye condition which is known as “congenital nystagmus”
[61]. Nystagmus and sensitivity to bright light may improve faintly.
Moreover, children may show paradoxical constriction of the pupil
in the dark, commonly have high hyperopic refractive errors (need
for plus power glasses). Although the fundus is usually normal,
in some affected individuals may be present macular changes
and vessel narrowing, retinal pigment epithelium disturbances
(separated into three categories: presence of retinal pigment
epithelium disturbance [50%], no retinal pigment epithelium
disturbance [27,5%], and atrophy [22,5%]), [62] or alteration of
the foveal reflex [63].
Diagnosis
The diagnosis of ACHM is established in a proband through
clinical and family history, examination for visual acuity testing,
nystagmus, fundoscopic examination, and color vision assessment.
If ACHM is suspected, supplementary tests will be recommended,
such as optical coherence tomography, fundus autofluorescence,
visual fields, and electroretinogram. Identification of biallelic
pathogenic variants in , CNGA3, CNGB3, GNAT2, PDE6C, PDE6H,
or ATF6 verify the clinical diagnosis. The family history is useful
to orientate the diagnosis by the inheritance mode. Family trees
are useful in these cases [64]. Visual field examination usually
demonstrates small central scotomas by careful testing, but this test
can be difficult to perform due to a lack of steady fixation [63]. The
color perception of individuals with achromatopsia (achromats) is
unreliable; many achromats learn to associate certain colors with
objects and to recognize some colors by discerning differences
in brightness [65]. Patients with achromatopsia demonstrate
defectiveness in all three axes of color vision. The most important
and diagnostic tests include the Rayleigh anomaloscope (which is the most reliable test), Farnsworth Munsell 100-Hue, and Panel
D-15 tests [66]. The most common electrophysiology tests used
in the diagnosis of achromatopsia are electroretinogram and
visual-evoked potentials [67,68]. This technique sometimes fails
to show pathologic changes in cases of achromatopsia due to a
limited number of photoreceptors within the fovea compared to
the entire retina [64]. A full-field ERG evaluates the whole retina.
The photopic response (including the 30-Hz flicker response) is
absent or notably diminished; the scotopic response is normal or
slightly abnormal. Genead [69] found that 75% of patients with
achromatopsia showed normal responses in this test.
Optical coherence tomography (OCT) is a great instrument
for the imaging and study of different structures in the eye.
Achromatopsia has also been studied with macular OCT [62].
Several clinical studies in recent years have investigated outer
retinal architecture and foveal morphology in detail using highresolution
optical coherence tomography (OCT) and adaptive
optics [62,70-77].
Sundaram categorized ACHM patients into the following five
groups based on outer retinal OCT findings (62).
a) continuous inner segment ellipsoid (Ise) (appeared in 22.5%
of the patients studied).
b) Ise disruption (27.5%).
c) absent Ise (20%).
d) foveal hyporeflective zone (22.5%).
d) outer retinal atrophy (7.5%).
No differences in the age, visual acuity, contrast sensitivity, or retinal sensitivity were found between patients in these five groups [62]. A variable degree of foveal hypoplasia as well as disruption and/or loss of inner-/outer-segment junction of the photoreceptors and an attenuation of the RPE layer within the macular region can be observed at an early age [62, 69, 72, 78,79]. Fundus autofluorescence is a relatively new imaging technique that takes advantage of the autofluorescent properties of lipofuscin to document its accumulation. Fahim described fundus autofluorescence in patients with achromatopsia [80]. Fundus autofluorescence imaging shows missing or variable formation of foveal hypofluorescence or a larger lesion with a surrounding hyperautofluorescent ring and a central region of absent autofluorescence corresponding to the lesion area seen on OCT [47,73]. Genetic testing can be useful for securing the diagnosis, risk assessment in relatives, prenatal diagnosis, and prognosis, and identifying carriers and new affected genes [66]. Molecular genetic testing approaches can include serial singlegene testing, use of a multi-gene panel, and more comprehensive genomic testing [66]. Targeted analysis for the most common pathogenic variant c.1148delC in CNGB3. The order for serial single-gene testing is based on the incidence of pathogenic variants in each gene. A multigene panel includes ATF6, CNGA3, CNGB3, GNAT2, PDE6C, PDE6H, and other genes of interest. Comprehensive genomic testing includes targeted exome [81], whole-exome, and whole-genome sequencing. This may be considered if other tests have not confirmed the diagnosis.
Treatment
There is usually no treatment to cure achromatopsia. In certain circumstances, patients can be worn red-tinted contact lenses or dark or special filter glasses to help reduce photophobia, increase brightness, improve visual acuity, and make it easier to identify colors, but many patients find them disorientating and difficult to wear. Tinted contact lenses supplemented with sunglasses or wrap-around glasses or use of opaque side-shields may also be used [82]. Tinted central contact lenses typically transmitting light at wavelengths between 400-480nm [83] are an improvement over simple cutoff filters in alleviating photophobia [82]. Additionally, these contacts have reduced the stigma of wearing dark wraparound sunglasses indoors. However, such red central contacts only decrease the amount of light entering the retina and do nothing to enhance high-resolution vision or color sensitive tasks. A partial solution is to use tinted contact lenses with magnification to boost what central vision is still present from functional photoreceptors [84]. If jurisdictional laws and visual acuity permit, patients with achromatopsia can drive a car with the use of bio-optics. Management targets symptoms and associated findings, striving to improve quality of life. Achromats frequently improve their own strategy that best works for them. Refractive errors should be checked (need for glasses). Prescribing glasses to correct refractive conditions such as far-sightedness (hyperopia), near-sightedness (myopia) and astigmatism can improve the vision somewhat but will not restore the normal levels of vision [10]. Children with achromatopsia should have preferential seating in the classroom (i.e., in the front to benefit maximally from magnifying devices and away from windows to reduce the effects of glare on vision) [6]. Consultation with low vision services would be valuable and often recommended to find appropriate low vision aids for school, work, or activities of daily living [10]. Psychosocial issues need to be also addressed with the patient and family. Low vision aids include high-powered magnifiers for reading as well as digital/electronic devices. There are small, handheld color detecting tools such as Colorino that allow detection of color for particular activities. These can be used to check the color of clothes. It is a color identifier that can identify more than 150 colors, announcing them in a clear voice in 20 different languages.
A newer device known as the Eyeborg system can help people with no color vision to perceive color through sound waves. Artist Neil Harbisson who suffered from achromatopsia was one of the first to use this device in early 2004 [10]. The color sensor in the Eyeborg detects colors, and then convert them to sounds, based on how high or low the pitch of the sound can provide a way to determine the color. Enormous efforts have been made in research in recent years to develop a possible cure for ACHM, and the new gene replacement therapeutic approaches are promising [85]. A specific vector is always required to deliver the target gene into the cell because DNA alone is not able to complete the task. Vector choice depends on the size of the cDNA of the type of targeted cells, the relevant gene, stability of expression, and immunogenicity [86]. Ongoing studies in mice, primates, and canines using adenoassociated virus (AAV) as vectors for gene therapy [86], have shown great promise. Three clinical Phase I/II safety trials for gene replacement therapy using Adenassociated viral vectors for achromatopsia association with CNGA3 and CNGB3 have been approved. A team from the Institute for Ophthalmic Research at the University Hospitals in Tübingen and the Departments of Pharmacy and Ophthalmology at LMU has developed a treatment that should, in principle, correct the CNGA3 genetic defect [87]. It involves introducing the normal version of the CNGA3 gene directly into the patient’s retina with the aid of a harmless virus, and hopefully the correctly functioning gene begins producing whatever protein was previously missing, helping cure the condition caused by the defective gene. The first clinical study of this approach in patients has just been completed at the University Eye Hospital Tübingen. In this study, nine patients with achromatopsia aged between 24 and 59 years were treated by injection of the virus bearing the intact CNGA3 gene into retina of the more severely affected eye [87]. The treatment can be classified as safe and potentially efficacious. The researchers mention the treatment will likely be most efficient if administered in childhood, as the brain is still highly plastic and the visual cortex has yet to fully develop. It is suggested in the study the ideal age may be before a patient reaches approximately seven years old as, after that point, the visual cortex has mostly developed and the primary structural loss of cone photoreceptors has taken place [88].
Objectives
The purpose of the thesis is to conduct a narrative review of achromatopsia and to examine the literature published to summarize the clinical disease, the genetic characteristics, and the recent advances in gene therapy. Specifically, this review will describe the management strategies with particular interest for the latest advances in gene therapy in humans.
Method of literature search
Study design
A literature review has been carried out to contribute to the scientific literature on achromatopsia and provide a comprehensive review of the clinical disease, the diagnosis and management of patients, and genetic characteristics. By means of a search on Pubmed, Scopus and Medline, 400 articles were obtained. The search was also the internet database for achromatopsia and gene therapy. The first step was oriented to articles published for congenital achromatopsia. The second step was focused on gene therapy achromatopsia. Papers published in any language during the last 20 years were selected. A scoring method regarding the scientific evidence (GRADE) was applied for all chosen manuscripts. Results: only 30 papers were considered as relevant for this review taking into account the research criteria.
Criteria for selection of publications
Inclusion criteria
Scientific articles published from January 2000 to February 2020, available without limitation to the language of their publication with regard to abstracts. Only meta-analyses, systematic reviews of the literature and controlled experimental studies in humans and animals that evaluated the gene therapy, and the diagnosis and management of patients with achromatopsia were selected. Filters like age, race, sex or geographical location were not taken into account.
Exclusion criteria
Articles that did not meet the inclusion criteria mentioned above were rejected.
Selection of articles
The selection of the articles was done analyzing the title, followed by reading the abstract to identify those that would be fully read. The exclusion and inclusion criteria expressed above were applied to the search results.
Result
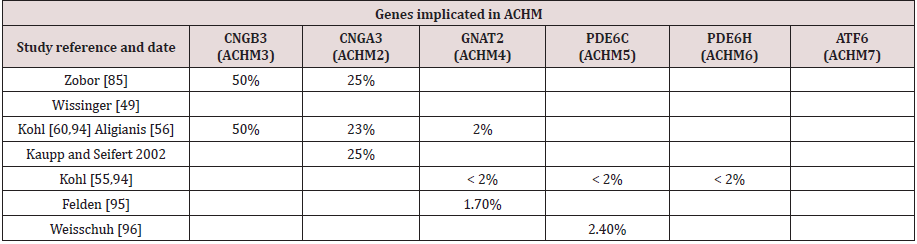
Genes identified as responsible for ACHM
To date, mutations in six different genes have been identified as responsible for ACHM: CNGA3, CNGB3, GNAT2, PDE6C, PDE6H, and ATF6 (Table 1&2).
Changes in retinal morphology
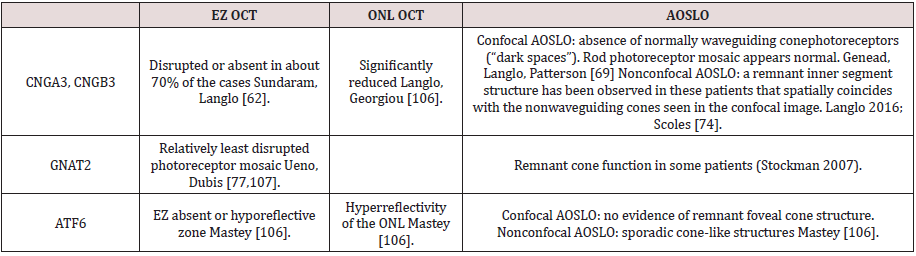
Optical coherence tomography (OCT) provides visualization of retinal lamination, enabling measurements of retinal layer thickness and intensity. Of particular interest are the hyperreflective (ellipsoid zone, EZ, and interdigitation, IZ) and hyporeflective (outer nuclear layer, ONL) bands associated with the photoreceptors [89-90]. Another imaging tool, adaptive optics scanning light ophthalmoscopy (AOSLO), enables noninvasive, cellular resolution imaging of the rod and cone photoreceptors mosaic. A confocal detector enables visualization of cones based on their waveguiding ability [91,92]. The waveguiding ability is thought to require intact outer segments [93] (Table 3).
Discussion
Clinically, approximately 50% of ACHM patients are associated with CNGB3 mutations, about 25% with CNGA3 mutations and smaller fractions with mutations in the cone transducing or phosphodiesterase genes in European populations [85-94]. GNAT2 mutations account for about 2% [48,49,56,94]. A study from Felden [95] estimated the prevalence of GNAT2-ACHM to be 1.7%, in a cohort of 1116 independent ACHM families [95]. GNAT2, PDE6C, and PDE6H, are each responsible for fewer than 2% of cases [55,94]. A recent study by Weisschuh [96] estimated the prevalence of the PDE6C genotype to be 2.4% in a cohort of 1074 independent ACHM families [96]. Mutations in ATF6 were recently identified in some subjects with ACHM who were negative for mutations in the aforementioned phototransduction genes [97,98]. In the ongoing debate about the stationary or progressive nature of ACHM, different genotypes should be studied individually. Achromatopsia has been classically described as stationary [99-101]; however, several recent studies have suggested it is a progressive condition. Thiadens [71] examined 40 ACHM patients using spectral-domain optical coherence tomography (SD-OCT), and reported that increased cone cell “decay” and retinal thinning was correlated with age, and began in early childhood [71]. Thomas [70], also showed progressive longitudinal changes in retinal morphology in children and described an interesting OCT sign, i.e. a hyperreflective zone which appears to be the precursor of the hyporeflective zone seen in later ages [70]. In contrast to these studies, Genead [69], provided evidence that cone loss is not age dependent [69]. Sundaram [62], examined 40 ACHM patients aged 6 to 52 years, and found no correlation between age and visual acuity, total foveal thickness, foveal ONL thickness, or inner segment ellipsoid (ISe) intensity and cone loss on SD-OCT [62]. Thiadens [71], in a cohort with five patients with PDE6 (20%) and no GNAT2, concluded that ACHM had been often a progressive disease [71]. PDE6CACHM was recently reported as a slowly progressive maculopathy [102]. ACHM studies conducted by Hirji [103] and Thiadens [71] with different proportions of patients with GNAT2 and PDE6C have shown conflicting results [71-103]. Hirji [103], in a cohort with one subject with PDE6C (2%) and two subjects with GNAT2 (4%), concluded that ACHM was predominantly stationary [103]. Although the retinal structure seems to show great variety and a slow progressive cone loss has been suggested [62,71,76,104,105], no clear association between retinal structure and function could be described [62]. Therefore, ACHM can be best described as a functional stationary disorder with slow degeneration of cone photoreceptors. Marked variability in the cone mosaic has been observed across patients; among the two most common genotypes, CNGA3 and CNGB3 [62, 69,102]. Carroll [75], observed a severely disrupted cone mosaic in the fovea and parafoveal area of a single patient with mutations in CNGB3 [75]. Genead [69], reported residual cone structures in all 9 patients carrying mutations in either CNGA3 or CNGB3, though the majority of cones had a reduced reflectance [69]. In contrast, in ATF6-ACHM and PDE6CACHM, few if any residual foveal cones were identified [102-106]. The GNAT2-ACHM genotype is associated with the relatively least disrupted photoreceptor mosaic [77-107].
Conclusion
Achromatopsia is a complex inherited disease. There is currently no approved treatment for ACHM. Management of achromatopsia is multifaceted, including making the appropriate diagnosis, reducing photophobia, correcting refractive error, and utilizing low vision devices, such as high-powered magnifying glasses. To reduce photophobia and potentially improving visual acuity can be helpful the use of dark or special filter glasses or red-tinted contact lenses. However, even with the best aid techniques, daily tasks such as going to school and driving present significant obstacles. New diagnostic and therapeutic tools are being studied. Fundus autofluorescence and OCT are important imaging techniques that supply significant information about the progression of the disease. Gene therapy is an ongoing therapeutic option already being studied in clinical trials. The new gene therapy being trialed centers on correcting a defect in the CNGA3 gene. The results of a first human trial testing for achromatopsia suggest that the experimental gene therapy is safe, and potentially efficacious, opening the door to larger human trials in the future. The evidence to date suggests that gene therapy will need to be applied early in childhood in order to achieve the best possible effect. The remarkable advances in genetics have opened an exciting world to ophthalmologists to offer patients new treatments for achromatopsia. In the future, gene therapy will be a routine treatment offered to patients.
References
- Genetics Home Reference (2015).
- Sacks O (1995) An Anthropologist on Mars Pp. 340.
- Pang JJ, Alexander J, Lei B (2010) Achromatopsia as a potential candidate for gene therapy. Adv Exp Med Biol 664: 639-646.
- Krill AE (1978) Krill’s Hereditary Retinal and Choroidal Diseases. Br J Ophthalmol 62(2): 130-131.
- Hess RF, Sharpe LT, Nordby K (1990) Total colour blindness. Cambridge: Cambridge University Press. United Kingdom.
- Kohl S, Jägle H, Wissinger B (2004) Gene Review.
- Jaeger W, Krastel H, Braun S (1988) Cerebral achromatopsia (symptoms, course, differential diagnosis, and strategy of the study). I. Klin Monbl Augenheilkd 193(6): 627-634.
- Bouvier SE, Engel SA (2006) Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cerebral Cortex 16(2): 183-191.
- Thiadens AAHJ (2011) Genetic Etiology and Clinical Consequences of Cone Disorders. Erasmus University Rotterdam.
- American Association for pediatric Ophthalmology and Strabismus (2019) San Francisco, United States.
- Brody JA (1970) Hereditary blindness among Pingelapese people of Eastern Caroline Islands. Lancet 295(7659): 1253-1257.
- Hussels IE (1972) Pingelap and Mokil Atolls: achromatopsia. Am J Hum Genet 24(3): 304-309.
- Morton NE (1972) Pingelap and Mokil Atolls: historical Am J Hum Genet 24(3): 277-289.
- Sundin OH (2000) Genetic basis of total colour blindness among the Pingelapese islanders. Nat Genet 25(3): 289-293.
- Molday RS, Moritz OL (2015) Photoreceptors at a glance. Journal of Cell Science 128: 4039-4045.
- Kazilek CJ, Cooper K (2010) Rods and Cones of the Human Eye. ASU, Ask A
- Longstaff A (2011) BIOS Instant Notes in (3rd edn.), London, United Kingdom.
- Williamson SJ, Cummins HZ (1983) Light and Color in Nature and New York, USA.
- Ahnelt PK, Kolb H, Pflug R (1987) Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. J Comp Neurol 255: 18-34.
- Ahnelt PK, Keri C, Kolb H (1990) Identification of pedicles of putative blue sensitive cones in human and primate retina. J Comp Neurol 293(1): 39-53.
- (2015) Cooper Vision. All the Different Kinds of Color Blindness.
- (2006) Tiresias.org. Guidelines: Color Blindness.
- Cassin B, Solomon S (1990) Dictionary of Eye Terminology. Gainsville, Florida: Triad Publishing Company.
- Pokorny J, Smith VC, Pinckers AJ, Cozijnsen M (1982) Classification of complete and incomplete autosomal recessive achromatopsia. Graefes Arch Clin Exp Ophthalmol 219(3): 121-130.
- Michaelides M, Hunt DM, Moore AT (2004) The cone dysfunction syndromes. Br J Ophthalmol 88(2): 291-297.
- Komáromy AM (2016) Emerging Treatments for Achromatopsia. Retinal Physician (13)46-56.
- Enchroma Types of Colour Blindness.
- Colour Blind Awareness. Types of Colour Blindness Nd.
- Jordan G, Mollon J (2019) Tetrachromacy: the mysterious case of extra-ordinary color vision. Behavioral Sciences 30: 130-134.
- New World Encyclopedia. Cone Cell Nd.
- Robinson J, Schmitt EA, Harosi FI, Reece RJ, Dowling JE (1993) Zebrafish ultraviolet visual pigment: Absorption spectrum, sequence and Proc Natl Acad Sci 90(13): 6009-6012.
- Remington LA, Goodwin D (2012) Clinical Anatomy and Physiology of the Visual System. (3rd edn). St. Louis: Elsevier/Butterworth-Heinemann p. 61-92.
- Arshavsky VY, Burns ME (2012) Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem 287(3): 1620-1626.
- Lamb TD, Pugh EN (2006) Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci 47(12): 5138-5152.
- Luo DG, Xue T, Yau KW (2008) How vision begins: an odyssey. Proc Natl Acad Sci 105(29): 9855-9862.
- Palczewski K (2014) Chemistry and biology of the initial steps in vision: The Friedenwald lecture. Invest Ophthalmol Vis Sci 55(10): 6651-6672.
- Arshavsky, Vadim Y, Lamb, Trevor D, Pugh, et al. (2002) G Proteins and Phototransduction. Annual Review of Physiology 64(1): 153–187.
- Burns ME, Pugh EN (2010) Lessons from photoreceptors: turning off G-protein signaling in living cells. Physiology (Bethesda) 25(2): 72-84.
- Korenbrot JI (2012) Speed, sensitivity, and stability of the light response in rod and cone photoreceptors: facts and models. Prog Retin Eye Res 31(5): 442-66.
- Pugh EN, Lamb TD (1993) Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta 1141(2-3): 111-49.
- Leskov IB, Klenchin VA, Handy JW, Whitlock GG, Govardovskii VI, et al. (2000) The Gain of Rod Phototransduction: Reconciliation of Biochemical and Electrophysiological Measurements. Neuron 27(3): 525–537.
- Farjo KM, Moiseyev G, Takahashi Y, Crouch RK, Ma JX (2009) The 11-cis-retinol dehydrogenase activity of RDH10 and its interaction with visual cycle Invest Ophthalmol Vis Sci 50(11): 5089-5097.
- Thompson DA, Janecke AR, Lange J (2005) Retinal degeneration associated with RDH12 mutations results from decreased 11-cis retinal synthesis due to disruption of the visual cycle. Hum Mol Genet 14(24): 3865-3875.
- Bartolomeo P, Bachoud-Lévi AC, Thiebaut MS (2014) The Anatomy of Cerebral Achromatopsia: A Reappraisal and Comparison of Two Case Cortex Elsevier 56: 138-144.
- Setälä K, Vesti E (1993) Acquired cerebral achromatopsia: A case report. Journal Neuro- Ophthalmology 14: 31-36.
- Kohl S, Marx T, Giddings I (1998) Total colourblindness is caused by mutations in the gene encoding the α-subunit of the cone photoreceptor cGMP-gated cation Nat Genet 19: 257-259.
- Kohl S, Zobor D, Chiang WC (2015) Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat Genet 47(7): 757-765.
- Kohl S, Varsanyi B, Antunes GA (2005) CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet 13(3): 302-308.
- Wissinger B, Gamer D, Jägle H, Giorda R, Marx T, et al. (2001) CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet 69(4): 722–37.
- Kohl S, Baumann B, Broghammer M, Jagle H, Sieving P, et al. (2000) Mutations in the CNGB3 gene encoding the beta-subunit of the cone photoreceptor cGMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome Hum Mol Genet 9: 2107-2116.
- Sundin OH, Yang JM, Li Y, Zhu D, Hurd JN, et al. (2000) Genetic basis of total colourblindness among the Pingelapese islanders. Nat Genet 25(3): 289-293.
- Rojas CV, Maria LS, Santos JL, Cortes F, Alliende MA (2002) A frameshift insertion in the cone cyclic nucleotide gated cation channel causes complete achromatopsia in a consanguineous family from a rural isolate. Eur J Hum Genet 10(10): 638-642.
- Ding XQ, Matveev A, Singh A, Komori N, Matsumoto H (2012) Biochemical characterization of cone cyclic nucleotide-gated (CNG) channel using the infrared fluorescence detection system. Adv Exp Med Biol 723: 769-775.
- Shuart NG, Haitin Y, Camp SS, Black KD, Zagotta WN (2011) Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion Nat Commun 2: 457.
- Kohl S, Coppieters F, Meire F, Schaich S, Roosing S, et al. (2012) A nonsense mutation in PDE6H causes autosomal-recessive incomplete achromatopsia. Am J Hum Genet 91(3): 527-532.
- Aligianis IA, Forshew T, Johnson S, Michaelides M, Johnson CA, et al. (2002) Mapping of a novel locus for achromatopsia (ACHM4) to 1p and identification of a germline mutation in the alpha subunit of cone transducin (GNAT2). J Med Genet 39(9): 656-660.
- Thiadens AA, den Hollander AI, Roosing S, Nabuurs SB, Zekveld-Vroon RC, et al. (2009) Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am J Hum Genet 85(2): 240-247.
- Chang B, Grau T, Dangel S, Hurd R, Jurklies B, et al. (2009) A homologous genetic basis of the murine cpfl1 mutant and human achromatopsia linked to mutations in the PDE6C gene. Proc Natl Acad Sci 106(46): 19581-19586.
- Grau T, Artemyev NO, Rosenberg T, Dollfus H, Haugen OH, et al. (2011) Decreased catalytic activity and altered activation properties of PDE6C mutants associated with autosomal recessive achromatopsia. Hum Mol Genet 20: 719-730.
- Kohl S, Varsanyi B, Autunes GA (2005) CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet 13(3): 302-308.
- Futterman F (2005) What Is Achromatopsia? 1997-2008.
- Sundaram V, Wilde C, Aboshiha J (2014) Retinal structure and function in achromatopsia: implications for gene therapy. Ophthalmology 121(1): 234-245.
- Remmer MH, Rastogi N, Ranka MP, Ceisler EJ (2015) Achromatopsia: a review. Curr Opin Ophthalmol 26(5): 333-340.
- Pascual Camps I, Barranco Gonzalez H, Aviñó Martínez J, Silva E, Harto Castaño M (2018) Diagnosis and Treatment Options for Achromatopsia: A Review of the Literature. J Pediatr Ophthalmol Strabismus 55(2): 85-92.
- Sharpe LT, Stockman A, Jagle H, Nathans J (1999) Opsin genes, cone photopigments, color vision, and color blindness. In: Gegenfurtner K, Sharpe LT(Eds.), Color Vision: from Genes to Perception Cambridge University Press Cambridge, UK, p.3-52.
- Kohl S, Jägle H, Wissinger B (2004) In: Adam MP, Ardinger HH, Pagon RA (Eds.), Gene Reviews. Seattle, Wa: University of Washington, Seattle 1993-2017.
- Whatham AR, Nguyen V, Zhu Y, Hennessy M, Kalloniatis M (2014) The value of clinical electrophysiology in the assessment of the eye and visual system in the era of advanced imaging. Clin Exp 92. Optom 97: 99-115.
- Young B, Eggen Berger E, Kaufman D (2012) Current electrophysiology in ophthalmology: a review. Curr Opin Ophthalmol 23(6): 497-505.
- Genead MA, Fishman GA, Rha J (2011) Photoreceptor structure and function in patients with congenital achromatopsia. Invest Ophthalmol Vis Sci 52: 7298-7308.
- Thomas MG, McLean RJ, Kohl S, Sheth V, Gottlob I (2012) Early signs of longitudinal progressive cone photoreceptor degeneration in Br J Ophthalmol 96: 1232-1236.
- Thiadens AA, Somervuo V, van den Born LI, Roosing S, van Schooneveld MJ (2010) Progressive loss of cones in achromatopsia: an imaging study using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 51(11): 5952-5957.
- Thomas MG, Kumar A, Kohl S, Proudlock FA, Gottlob I (2011) High-resolution in vivo imaging in achromatopsia. Ophthalmology 118: 882-887.
- Greenberg JP, Sherman J, Zweifel SA, Chen RW, Duncker T (2014) Spectral-domain optical coherence tomography staging and autofluorescence imaging in achromatopsia. JAMA Ophthalmol 132(4): 437-445.
- Scoles D, Sulai YN, Langlo CS, Fishman GA, Curcio CA, (2014) In vivo imaging of human cone photoreceptor inner segments. Invest Ophthalmol Vis Sci 55: 4244-4251.
- Carroll J, Choi SS, Williams DR (2008) In vivo imaging of the photoreceptor mosaic of a rod monochromat. Vision Res 48(26): 2564-2568.
- Aboshiha J, Dubis AM, Cowing J, Fahy RT, Sundaram V, et al. (2014) A prospective longitudinal study of retinal structure and function in Invest Ophthalmol Vis Sci 55(9): 5733-5743.
- Dubis AM, Cooper RF, Aboshiha J, Langlo C, Sundaram V, et al. (2014) Genotype-dependent variability in residual cone structure in achromatopsia: towards developing metrics for assessing cone health. Invest Ophthalmol Vis Sci 55: 7303-7311.
- Lee H, Purohit R, Sheth V, McLean RJ, Kohl S, et al. (2015) Retinal development in infants and young children with achromatopsia. Ophthalmology 122: 2145-2147.
- Zobor D, Werner A, Stanzial F, Benedicenti F, Rudolph G, et al. (2017) The clinical phenotype of CNGA3-related achromatopsia: pretreatment characterization in preparation of a gene replacement therapy trial. Invest Ophthalmol Vis Sci 58(2): 821-832.
- Fahim AT, Khan NW, Zahid S (2013) Diagnostic fundus autofluorescence patterns in achromatopsia. Am J Ophthalmol 156(6): 1211-1219.
- Li FF, Huang XF, Chen J (2015) Identification of novel mutations by targeted exome sequencing and the genotype-phenotype assessment of patients with achromatopsia. J Transl Med 13: 334.
- Schornack MM, Brown WL, Siemsen DW (2007) The use of tinted contact lenses in the management of achromatopsia. Optometry 78: 17-22.
- Park WL, Sunness JS (2004) Red contact lenses for alleviation of photophobia in patients with conedisorders. Am J Ophthalmol 137: 774-775.
- Fonda G, Thomas H (1974) Correction of low visual acuity in achromatopsia. Use of corrective lenses asan aid to educational and vocational placement. Arch Ophthalmol 91: 20-23.
- Zobor D, Zobor G, Kohl S (2015) Achromatopsia: On the Doorstep of a Possible Therapy. Ophthalmic Res 54: 103-108.
- Roosing S, Thiadens AA, Hoyng CB, Klaver CC, den Hollander AI, et al. (2014) Causes and consequences of inherited cone disorders. Prog Retin Eye Res 42: 1- 26.
- 2020 Ludwig Maximilian University of Munich. New gene therapy for complete color blindness tested in patients. Medical Xpress.
- Haridy R (2020) Gene therapy for color blindness passes first phase of human testing. New Atlas.
- Spaide RF, Curcio CA (2011) Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina 31(8): 1609-1619.
- Jonnal RS, Kocaoglu OP, Zawadzki RJ, Lee SH, Werner JS, et al. (2014) The cellular origins of the outer retinal bands in optical coherence tomography images. Invest Ophthalmol Vis Sci 55(12): 7904-7918.
- Dubra A, Sulai Y, Norris JL (2011) Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed Opt Express 2(7): 1864-1876.
- Roorda A, Duncan JL (2015) Adaptive optics ophthalmoscopy. Annu Rev Vis Sci 1: 19-50.
- Roorda A, Williams DR (2002) Optical fiber properties of individual human cones. J Vis 2(5): 404-412.
- Kohl S, Baumann B, Broghammer M (2002) Mutations in the cone photoreceptor G-protein α-subunit gene GNAT2 in patients with Am J Hum Genet 71(2): 422-425.
- Felden J, Baumann B, Ali M (2019) Mutation spectrum and clinical investigation of achromatopsia patients with mutations in the GNAT2 gene. Hum Mutat 40(8): 1145-1155.
- Weisschuh N, Stingl K, Audo I (2018) Mutations in the gene PDE6C encoding the catalytic subunit of the cone photoreceptor phosphodiesterase in patients with achromatopsia. Hum Mutat 39: 1366-1371.
- Ansar M, Santos Cortez RL, Saqib MA (2015) Mutation of ATF6 causes autosomal recessive achromatopsia. Hum Genet 134(9): 941-950.
- Skorcyzk Werner A, Chiang WC, Wawrocka C (2017) Autosomal recessive cone-rod dystrophy can be caused by mutations in the ATF6 gene. Eur J Hum Gen 25(11): 1210-1216.
- Michaelides M, Hunt DM, Moore AT (2004) The cone dysfunction syndromes. Br J Ophthalmol 88(2): 291-297.
- Andréasson S, Tornqvist K (1991) Electroretinograms in patients with achromatopsia. Acta Ophthalmol 69(6): 711-716.
- Johnson S (2004) Achromatopsia caused by novel mutations in both CNGA3 and CNGB3. J Med Genet 41(2):
- Georgiou M, Litts KM, Katitzeos A (2019) Adaptative optics retinal characterization, interocular symmetry, and intrafamilial variability. Invest Ophthalmol Vis Sci 60(1): 383-396.
- Hirji N, Aboshiba J, Georgiou M, Bainbridge J, Michaelides M (2018) Achromatopsia: clinical features, molecular genetics, animal models and therapeutic options. Ophthalmic Genet 39(2): 149-157.
- Wissinger B, Schaich S, Baumann B, Bonin M, Jägle H, et al. (2011) Large deletions of the KCNV2 gene are common in patients with cone dystrophy with supernormal rod Hum Mutat 32(12): 1398-1406.
- Komaromy AM, Rowlan JS, Corr AT, Reinstein SL, Boye SL, et al. (2013) Transient photoreceptor deconstruction by CNTF enhances rAAV-mediated cone functional rescue in late stage CNGB3- achromatopsia. Mol Ther 21(6): 1131-1141.
- Mastey RR, Georgiou M, Langlo CS (2019) Characterization of retinal structure in ATF6-associated achromatopsia. Invest Ophthalmo Vis Sci 60(7): 2631-2640.
- Ueno S, Nakanishi A, Kominami T (2017) In vivo imaging of a cone mosaic in a patient with achromatopsia associated with a GNAT2 variant. Jpn J Ophthalmol 61: 92- 98.