Lupine Publishers Group
Lupine Publishers
Case Report(ISSN: 2644-1209) 
A Case and Cranial MRI Characteristics of Progressive Supranuclear Palsy Volume 2 - Issue 1
Asuman Ali1, Cemile Haki2, Oğuzhan Gümüştaş3*, Ersin Budak4 and Ramazan Yalçın5
- 1,2Department of Neurology, Turkey
- 3,5 Department of Radiology, Turkey
- 4Department of Psychiatry, Turkey
Received: December 05, 2018; Published: December 13, 2018
*Corresponding author: Oğuzhan Gümüştaş, Department of Radiology, Radiological Diagnosis Center, Bursa, Turkey
DOI: 10.32474/TOOAJ.2018.02.000128
Abstract
Progressive supranuclear palsy (PSP) is a neurodegenerative disease described by vertical supranuclear gaze paralysis, axial rigidity and postural instability involving falls in the early stage of the disease. Imaging studies present brain atrophy in especially midbrain, premotor cortex, basal ganglia and thalamus. There is no bioindicator of the disease although it is clinically identified. PSP is also classified as tauopathy. However, no specific indicator for tau protein could be identified. Hence, imaging findings, especially midbrain atrophy, functions as a biomarker in clinical practice and determination of disease prognosis. It has been shown that midbrain atrophy has a diagnostic value for PSP accompanied with the finding of “hummingbird” or “penguin” encountered by magnetic resonance imaging (MRI).
In this case report, we have aimed to review the diagnostic features of the disease based on the results of clinical and radiological results and to demonstrate the “penguin” sign and midbrain atrophy as a biomarker for clinical follow-up process. We have also detected a correlation with the results of clinical and neuropsychological tests.
We have analyzed a 61-year-old male patient who was a retired teacher for the complaints of progressive difficulty in walking since the recent year, slowness in speech fluency, forgetfulness and easy falling. Our patient was performed 1.5 Tesla Cranial MRI study due to the signs of postural instability, easy falling backward while walking, cognitive impairment, apathia, conjugate vertical gaze palsy, disinhibition and anxiety. T1W, T2W, T2 flair and diffusion sections on the axial plane were obtained in this imaging study. In addition, Mini-Mental State Examination and neuropsychological test were also performed in our patient.
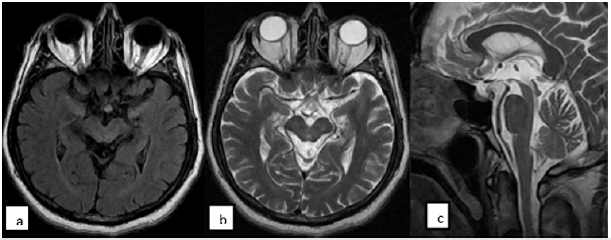
The patient had particularly diffuse cerebral atrophy in predominantly frontal and parietal lobes. Peripheral CSF spaces were enlarged due to atrophy. Brainstem also showed atrophy and particularly mesencephalon atrophy was more remarkable. The appearance of brainstem in the sagittal slices due to mesencephalon atrophy were termed as “penguin” sign. A mild atrophy was also encountered in the bilateral cerebellar hemispheres. As a result of neuropsychological evaluation, mild to moderate cognitive impairment were detected.
We have evaluated with this case that atrophy of midbrain and mesencephalon which presented a significant visuality with classic “penguin” sign has an important diagnostic value in the PSP cases. Additionally, we have reviewed the fact in the light of literature data that imaging of midbrain atrophy may play a role as a biomarker in performing clinical follow-up and determination of the prognostic features of the PSP cases.
Introduction
Progressive supranuclear palsy (PSP) is a neurodegenerative disorder of primitive tauopathies [1]. It is characterized with pseudobulbar paralysis, dysarthria, dystonic rigidity of neck and trunk, akinesia, postural instability involving frequent falls and frontotemporal dementia as well as vertical gaze paralysis [2]. PSP is the most commonly seen atypical Parkinsonism. Its prevalence is 6.0-6.4 in 100,000. Its incidence is 5.4 cases in 100,000 [3]. It should be differentiated from idiopathic Parkinson’s disease or other atypical Parkinsonism cases along its natural clinical course [4,5]. The neuropathologically determinant characteristic of PSP is midbrain atrophy.
Atrophy is associated with intensity of tau-positive aggregates which are not melted in any way. These tau-positive formations are neuronal and glial fibrillar tangles and neurophil threads [6- 8]. This abnormal accumulation of tau proteins are responsible for the neuronal degeneration of basal ganglia and midbrain nuclei. Midbrain atrophy involves tegmentum, tectum, quadrigeminal layer and periaqueductal gray matter and is associated with reduced intensity of myelinated fibers, gliosis and neuronal loss. It is more commonly observed in males. Retropulsion and postural instability observed at early stage lead to frequent falls. The occurrence of the falls within the initial onset year is a typical characteristic. On the other side, supranuclear gaze paralysis usually appears due to primarily slowing of vertical saccades. Dystonia in the frontalis and procerus muscles may give a facial expression of scared or surprised in the PSP patient. Different clinical types of PSP have been recently identified [6]. In PSP patients, especially attention, executive function loss, verbal and nonverbal memory deficiencies may develop. The performance of the patient is demonstrated by verbal fluency tests, Frontal Assessment Battery and Montreal Cognitive Assessment Battery [4,9].
Case
The 61-year-old male patient was evaluated for the complaints of difficulties in starting walking since the recent year, frequent backward falls while walking, slowed speech and eye opening apraxia. He was conscious however, he had slow speech fluency and hypophonic dysarthria. His blink reflex was reduced. He had upward and downward supranuclear gaze paralysis and axial rigidity. He was able to walk independently with unsafe wide steps in the short distance. “Pull” test result was positive. His emotional state was labile and a remarkable slowing started in the executive functions. No response to the treatment of L-Dopa could be obtained.
Methods
The case was performed 1.5 Tesla MRI study. Cranial MRI was performed in accordance with T1weighted axial plane (slice thickness:5.5mm;TR:560;TE:9.5, Matrix 288x182), T2weighted sagittal plane (slice thickness: 5mm, TR: 5300, TE:82, Matrix: 384x192), T2weighted flair on the axial plane (slice thickness:5.5 mm,TR:8000, TE: 98, TI:2000, Matrix:320x224); T2weighted axial plane (slice thickness: 5.5mm,TR:3700, TE:88 Matrix: 320x256) (Figure 1). In addition, Montreal Cognitive Assessment Battery (MOCA Test) was applied. “Benton” facial recognition test and Line Direction Tests were performed.
Discussion and Conclusions
PSP is a clinical diagnosis. There is no diagnostic laboratory test. Midbrain atrophy that is frequently encountered by Cranial MRI may play a role as biomarker in determination of the prognosis. Especially, image of “penguin” encountered in the sagittal MRI slices has a diagnostic value. The progression of Parkinson’s Disease which is one of the diseases to be considered in differential diagnosis is very much slower than PSP and has no “red flag symptoms” and its responsiveness to L-Dopa is very well [1-4].
Corticobasal degeneration is a tauopathy like PSP, however, it is characterized with Parkinsonism manifested by remarkable symmetric dystonia. It is accompanied by cortical sensory signs, progressive apraxia is typically observed and “Alien Hand Syndrome” occasionally appears. It is differentiated by autonomic involvements such as Multiple System Atrophy with orthostatic hypotension, cerebellar ataxia and inspiratory stridor. Parkinson’s-Dementia complex manifested by Lewy body dementia and Parkinsonism is differentiated by fluctuating mental state and visual hallucinations appearing with onset of dopaminergic treatment [1-6].
Cranial MRI of the case encountered diffuse cerebral atrophy in predominantly frontal and parietal lobes. Peripheral cerebrospinal fluid (CSF) spaces were enlarged due to atrophy. Atrophy was present also in the brainstem and the atrophy particularly in the mesencephalon is much more remarkable. The appearance of the brainstem due to mesencephalon atrophy in the sagittal slices was termed as “penguin sign”. Additionally, bilateral cerebellar hemispheres revealed a slight atrophy [7-9].
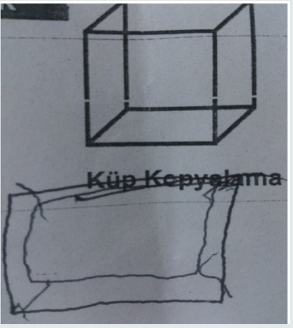
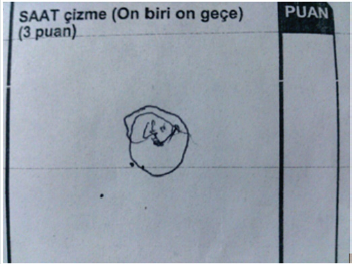
According to MOCA Test scores, a significant decline was monitored in the copying and visuospatial functions of the patient. The patient gained no point from the subfield of executive functions. He has experienced difficulties in ordering the parameters in a correct sequence in this subfield. He could not order the parameters (1-5, A-E) as a number followed by a letter although he was a retired teacher. This result indicated a significant problem in the executive functions of this patient. In this subfield, the patient could not copy a cube and could gain no point from the subfield of clock-drawing test (Figures 2 & 3). The patient gained 5 standard deviations lower than the mean of his age and educational level according to the result of Benton Test of Facial Recognition. He was found significantly unsuccessful in Line Direction Tests. Evaluation of visual performance tests: It was observed that the patient’s cognitive processes related to orientation, spatial perception, spatial cognition, mental rotation, and face recognition were significantly impaired.
References
- Ferri Fred F (2014) Ferri’s Clinical Advisor 2015 by Mosby. Progressive Supranuclear Palsy pp. 985-985.
- Tessitore A, Giordano A, Caiazzo G, Corbo D, et al. (2014) Clinical correlations of microstructural changes in progressive supranuclear palsy. Neurology of Aging 35(10): 2404-2410.
- William DR, Lees A (2009) Progressive Supranuclear Palsy: clinicopathological concepts and diagnostic challenges .Review. Lancet Neurol 8(3): 270-279.
- Bloise MC, Berardelli l, Roselli V, Pasquini M, Stirpe P et al. (2014) Psychiatric disturbances in patients with PSP: A case-control study. Parkinsonism Relat Disord 20(9): 965-968.
- Barsottini OGP, Ferraz HB, Maia AC, Silva CJ, Rocha AJ (2007) Differentiation of Parkinson’s disease and progressive supranuclear palsy with magnetic resonance imaging: The first Brazilian experience. Parkinsonizm and Related Disorders 13(7): 389-393.
- Aralasmak A, Koçak M (2010) Imaging in Neurodegenerative Disorders. Semin Roentgenol 45(2): 126-136.
- Tartaglia MC (2012) Frontotemporal lobar degeneration: New Understanding Bring new Approaches. Neuroimaging Clin N Am 22(1): 83-97.
- Ling H,Lees AJ (2010) How can Neuroimaging Help in the Diagnosis of Movement Disorders? Neuroimaging Clin N Am 20(1): 111-123.
- Bote RP, Fernandes-Gil MA (2013) Degeneration of the Brainstem. Semin Ultrasound CT MRI 34(2): 142-152.