Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Review Article(ISSN: 2637-6660) 
Understanding the Machinery of Mycobacteriophage to Control Infection of Mycobacterium tuberculosis H37Rv Using Mycobacterium Smegmatis Volume 2 - Issue 2
Manas Gupta, Shivangi and Laxman S. Meena*
- CSIR-Institute of Genomics and Integrative Biology, India
Received: November 08, 2019; Published: December 06, 2019
*Corresponding author: Laxman S Meena, CSIR-Institute of Genomics and Integrative Biology, India
DOI: 10.32474/ANOAJ.2019.02.000133
Abstract
Mycobacterium tuberculosis H37Rv (M. tuberculosis) infection is one of the most common and deadliest infection in present time and the main reason of mortality due to an infectious disease i.e. Tuberculosis (TB). Mycobacteriophage therapy is a novel study for controlling this bacterial infection. Mycobacteriophage are the member of a group of bacteriophages that infects Mycobacterium and are specific for their host. Infection on Mycobacterium result in their lysis making Mycobacteriophage a viable topic for study, the infection requires a number of proteins to lyse the cell wall core i.e. Mycolyl-arabinogalactan peptidoglycan complex of the bacterium and other layers of cell envelope. This review article highlights crucial points about lytic proteins synthesized by Mycobacteriophage Ms6 and the factors like pH, temperature, phage titre, etc. which affect the infection and growth of phage inside the M. tuberculosis. Further article talks about in-vivo infection strategy used to infect Mycobacterium avium109 (M. avium) using a non-virulent strain of Mycobacterium infected with TM4 mycobacteriophage. Thus, this article provides a basic fundamental knowledge about the working mechanism of mycobacteriophage that help to control or reduce the infection of M. tuberculosis.
Keywords: Mycobacterium tuberculosis; Mycobacteriophage Ms6; Mycobacteriophage TM4
Abbreviation: tuberculosis: Mycobacterium tuberculosis H37Rv; TB: Tuberculosis; HIV: Human Immunodeficiency Virus ; M. smegmatis : Mycobacterium smegmatis; DNA: Deoxyribonucleic acid; AIDS: Acquired Immune Deficiency Syndrome; MOI: Multiplicity Of Infection; Ca2+: Calcium Ion; Lys B: lysin B ; M. avium : Mycobacterium avium109; MBTC: Mycobacterium Tuberculosis Complex; mAGP: Mycolyl-Arabinogalactan Peptidoglycan; M. bovis: Mycobacterium bovis; pNPB: p-Nitrophenyl Butyrate; Mn2+: Manganese Ion; Mg2+: Magnesium Ion; K+: Potassium Ion; PMSF: Phenyl Methyl Sulfonyl Fluoride; WHO: World Health Organisation
Introduction
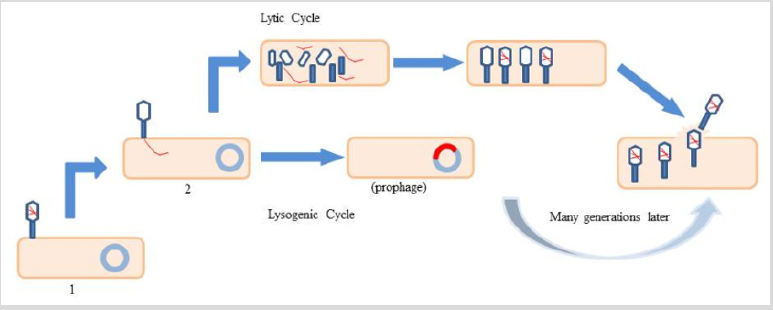
One of the oldest but still one of the biggest human infectious afflictions is Tuberculosis (TB) which is mainly caused by Mycobacterium tuberculosis H37Rv (M. tuberculosis) [1]. One of the major reasons behind the increasing number of mortal cases due to this affliction is bacterial acclimatization in human alveolar macrophages [2]. Bacteriophages are among the most common and diverse entities in the biosphere. A bacteriophage also known informally as a phage or a virus that infects and replicates within bacteria [3]. A Mycobacteriophage is a member of a group of bacteriophages known to infect mycobacteria as their host bacterial species. They have played an important role in decreasing infection of Mycobacterium by developing tools against molecular genetics of bacteria [4]. These phages were originally isolated from the various members of Mycobacterium family [5]. Mycobacteriophages head is composed of protein capsid which encapsulates either DNA or RNA. The oval head is connected to a distal end of the tail, through a neck or connector region at the head-tail junction [6] (Figure 1). To replicate inside the bacterium, phages can employ two pathways i.e. Lytic and Lysogenic cycle (Figure 2). In lytic cycle, bacterial cells are broken open (lysed) and destroys the bacterium cells after immediate replication of the virions. As soon as the cell is destroyed, the phage progeny can find new surrounding cells to infect. In contrast, the lysogenic cycle does not result in immediate lysing of the host cell. Those phages able to undergo lysogenic are known as temperate phages [7]. Their viral genome integrate with host DNA i.e. prophage, and replicate along with it, relatively harmlessly, or may even become established as a plasmid. The virus remains dormant until host conditions deteriorate; perhaps due to depletion of nutrients, then the endogenous phages (prophage) become active. At this point they initiate the reproductive cycle, resulting in lysis of the host cell. As the lysogenic cycle allows the host cell to continue to survive and reproduce, the virus is replicated in all offspring of the cell [8]. Mycobacteriophages genome contains genes that perform various function such as Integration, DNA replication, recombination, and modification, DNA packaging and lysis and Virion formation and assembly [9].
Figure 1: Diagrammatic view of Mycobacteriophage: A typical Mycobacteriophage’s body is divisible in head and tail. The head consist of either DNA or RNA, encapsulated by protein capsid. The tail consists of a tail tube and baseplate by which it attaches to its host. The head and tail are connected to each other via a connector neck.
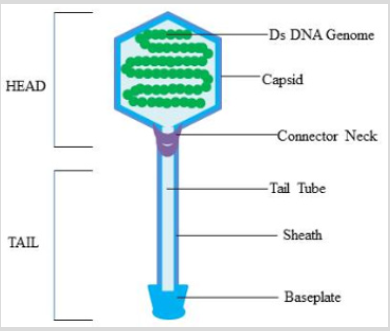
Figure 2: Lytic and lysogenic cycle of a typical Bacteriophage: There are several stages. 1- Attachment of bacteriophage, 2-bacteriophage releases its genetic material DNA or RNA inside bacterium. After this, bacteriophage can go for either lytic or lysogenic cycle. In lytic cycle it uses the host machinery to synthesize its protein coat and other components , later it assembles itself and finally lyse the cells releasing the progeny, or bacteriophage can go for lysogenic cycle where in lysogenic cycle bacteriophage’s genetic material gets incorporated into bacterium genome which is known as prophage later it shifts to lytic phase.
Overview of Mycobacteriophage Ms6
The lytic cassette of Mycobacteriophage Ms6, a temperate phage that infects Mycobacterium smegmatis (M. smegmatis) comprises of five genes. Endolysins that hydrolyses the peptidoglycan (Figure 3) when applied externally to bacterial cell wall, resulting in rapid lysis of the bacterial cell and Holin protein – a small membrane protein that defines the time of lysis and allows phage endolysins to cleave the target. Recent results have shown that endolysins may be transported across the cytoplasmic membrane in a holing independent manner. The gene lysA encodes the endolysins, while the holin is encoded by Gp4 (hol). The Gp1 and Gp5 genes code for proteins of unknown functions. It was observed that co-expression of Gp1 and lysA in Escherichia coli (E. coli) results in cell lysis and no such effect occurs when only one of the two genes was independently expressed. This led to investigation the role of the Gp1 gene product in lysis. Experimental evidence shows that Ms6 Gp1 is involved in lysis assisting the export of endolysins to the extra cytoplasmic environment by acting like a chaperone-like protein i.e. Gp1 protein interacts endolysins [10]. In addition to these to proteins Mycobacteriophage Ms6 also codes for a protein known as LysB. Analysis of the LysB, deduced amino acid sequence has revealed the presence of a conserved penta-peptide Gly-Tyr-Ser-Gln-Gly motif, which matches the Gly-X-Ser-X-Gly consensus motif that is characteristic for lipolytic enzymes [11].
The cell wall of M. smegmatis is a gram-positive bacterium but has a complex cell wall structure consisting of peptidoglycan covalently linked with arabinogalactan which is esterified to variety of long chain fatty acids known as mycolic acids. This mycolyl-arabinogalactan peptidoglycan (mAGP) is known as cell wall core [12]. An experiment was performed to see the activity of LysB on mAGP which revealed that the non-reducing termini of penta-arabinosyl motifs in the arabinogalactan are esterifies to mycolic acids. The release of free mycolic acid residues by the action of LysB demonstrated that LysB acts on ester bond between mycolic acid and arabinogalactan, thereby disrupting the inner leaflet of mycobacterial outer membrane [13] (Figure 3). By further analysis it was revealed that the lipid profile changes after LysB treatment on total extractable lipids from M. smegmatis. Interestingly the same result is observed with total lipids from other species of Mycobacterium. This indicates that LysB is not specific for M. smegmatis, as it is able to hydrolyze lipids from other mycobacterial species [14].
Figure 3: Action of Endolysins and LysinB protein on cell envelope of a typical Mycobacterium: The lysB protein acts on the mAGP complex and Endolysin acts on peptidoglycan helps in cleaving the mycobacterial cell membrane which finally kills bacterium; CL: Complex Lipids; MA: Mycolic Acid; AG: Arabinogalactan; PG: Peptidoglycan; CM: Cell Membrane.
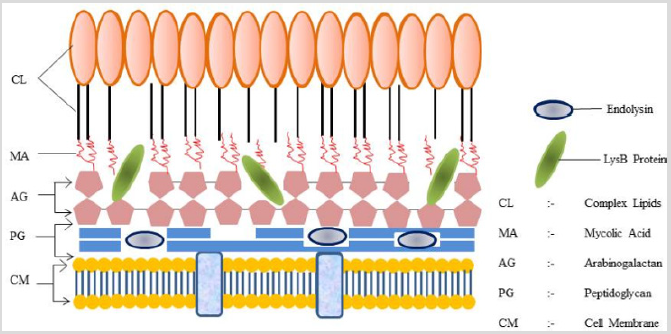
Experimentation on LysB by using p-nitrophenyl butyrate (pNPB) as the substrate revealed that the activity of LysB protein displayed temperature dependence as the highest specific activity was achieved at 23°C although a high level of residual activity (>72%) was observed in the tested temperatures (4-63°C). The enzyme shows maximum activity at pH 7.5-8.8 and maintains the activity above 50% in the pH range 6.8-9.5. Almost no activity was observed at pH 5 (2.2%). The presence of divalent ions like Ca2+ or Mn2+ increased the activity of protein, whereas the addition of Mg2+ and K+ made no significant difference on the activity of the LysB protein. Complete inhibition was obtained in the presence of PMSF, supporting the notion that a LysB protein is a serine protease [11].
In-vivo infection on avium using M. smegmatis infected TM4 phage cells
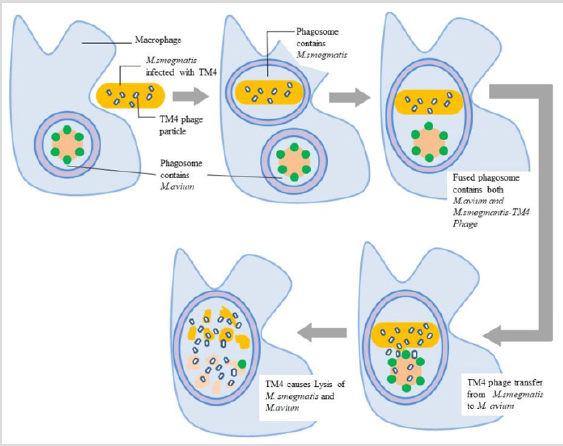
TM4 is a Mycobacteriophage which is known to cause infection in Mycobacterium avium 109 (M. avium), as this is causative agent for tuberculosis in people suffering from AIDS. Moreover, M. avium was selected for Mycobacteriophage experiments because of its ability to infect above mentioned strain of Mycobacterium tuberculosis complex (MTBC) [15]. In-vitro when the phage titre was mixed with the media containing M. avium, the results were satisfactory i.e. the reduction in number of bacilli was observed [16]. Although same behavior was not observed in in-vivo condition seemingly because the macrophages didn’t engulf the Mycobacteriophage particle or the macrophages which had M. avium did not go further for the phagocytosis of TM4 Phage particles. To overcome this complication a new strategy was employed i.e. to utilize a non-virulent species as a vehicle to deliver the phage particle to virulent strain. The central idea is to propagate TM4 phage particle inside the non-virulent species which is in this case is M. smegmatis that would deliver the phage particle to the phagosome infected with M. avium. This co-infection of M. avium and M. smegmatis transiently infected by TM4 phage could result in fusion of infected vacuoles consecutively delivering the phage particles to M. avium (Figure 4). Subsequently the results were obtained from an experiment that displayed a 10-fold decrease when macrophages were treated with M. smegmatis-TM4 after 24 h of infection; a 100-fold decrease in the number of intracellular M. avium was seen as the result of treatment of infection with M. smegmatis-TM4 [15]. To ensure fusion of infected vacuoles the sample was subjected to real-time video microscopy as M. avium grown to logarithmic phase is a short bacilli and M. smegmatis is always a long rod, it was feasible to follow the dynamics of the infection daily by using the above method [17].
Figure 4: Infection on M. avium using M. smegmatis-TM4 Infected Cells: This figured depicts how M. smegmatis is used to infect M. avium in in-vivo conditions. Image shows that infected macrophage with M. avium engulfs M. smegmatis - TM4 Phage bacterium, the infected vacuoles fuse to form one phagosome, later TM4 phage inside phagosome infects M. avium bacterium and ultimately lyse bacteria.
Factors affecting infection of mycobacteriophage on mycobacterium
Infection on Mycobacterium requires that the
Mycobacteriophage adsorbs by the host alveolar macrophage.
Adsorption of a phage to its host cell is majorly dependent on
the electrostatic forces between cell and virus; the net repulsive
force created by an excess of negative charges on both phage and
host cell that can be neutralized either by double-layer formation
of cations in the suspending medium [18]. Important factor that
affects the propagation of Mycobacteriophages is the presence
of Ca2+ ions, present as CaCl2 in the environment. Ca2+ has the
capacity to promote the entry of phage DNA into the host cell and
thus initiate an infection cycle [19,20]. Adsorption was increased
from 10% to 20% by the incorporation of either Ca2+, Sr2+, Mg2+or
Zn2+ in the medium. Fewer phage particles were adsorbed in the
presence of K+ or Na+ than both alone [21]. The ferrous compounds
tested were highly effective and could inactivate virus particles
at concentrations down to 5mM. FAS was required for the full
inactivation of phage suspensions, phage that had infected host
bacteria were able to survive treatment with FAS [22].
The optimum temperature at which Mycobacteriophage grows
is 37°C, supported experimentally by using a temperature-sensitive
mutant (D29ts10) which does not grow at 42°C, but seems to do so,
although less efficiently than its wild type i.e. mycobacteriophage
D29 at 37°C. The results show that this mutant fails to kill the
cells at 42°C. But at 37°C cell killing was observed, although to a
lesser extent. In addition it was observed that the concentration
of phage in phage titre also plays an important role in infection. It
was concluded that there was a time delay for the onset of death
because the starting phage concentration was lower than that of
the bacteria e.g. Multiplicity of infection (MOI) of 0.1. The time
delay appears to be necessary for the phage titres to increase the
minimum value of MOI to 1 [23]. To combat infection of bacterium,
this strategy therefore provides an important path to tackle the
route of pathogenesis in a clear and easy way.
Conclusion
Efforts have been made from decades to reduce pathogenesis level of Mycobacterium tuberculosis [24]. World Health organization (WHO) reports since last two- three years made an alert situation from this disease [25]. Although number of mortal and incidences cases have been reduced in last year’s but it should reduced with greater extent in accordance with the population of the country [26,27]. Mycobacteriophage infection on Mycobacterium causes lysis of bacterium. The growth of Mycobacteriophage is affected by various factors like temperature, pH and titre phage, presence or absence of certain factors, compounds, and ions in the surrounding. The lysis of bacterium by infection of Mycobacteriophage is achieved as Mycobacteriophages releases some proteins that lyse the cell wall core and the cell envelope. These lytic proteins are coded by Mycobacteriophage Ms6 consisting of 5 genes. Gene product of Gp1 acts as a chaperone-like protein interacts with the endolysins protein and lyses the peptidoglycan of the cell wall. The protein lysB helps in lysing the cell wall core i.e. mAGP complex of the Mycobacterium, Holin protein in phage determines the time of lysis of the bacterium. Furthermore in in-vivo conditions, M. smegmatis (Non-virulent-strain) infected with TM4 Mycobacteriophage can be used to deliver the TM4 phage to M. avium (Virulent-strain) which further causes lysis of M. avium, this can be considered as strategy of infection as 1° host of TM4 in a non-virulent strain which pass on the phage particle to the 2° host a virulent one. By employing the proteins mentioned above as well as using the in-vivo infection strategy of Mycobacteriophage over Mycobacterium species, the role of their involvement to lower the infection could be better understanding.
Highlights
a) Mycobacteriophages are the member of a group
of bacteriophages that infects Mycobacterium.
b) They utilize two cycles to kill their host i.e. via lytic and
lysogenic.
c) Growth of Mycobacteriophage is affected by various
factors like temperature, pH, and titre phage and ions.
d) Endolysin and lysB proteins play major role in disrupting
cell wall envelope of the bacterium.
References
- Meena LS, Rajni (2011) Survival mechanisms of pathogenic Mycobacterium tuberculosis The FEBS J 277 (11): 2416-2427.
- Shivangi, Meena LS (2018) A novel approach in treatment of tuberculosis by targeting drugs to infected macrophages using biodegradable nanoparticles. Appl Biochem Biotechnol 185(3): 815-821.
- McGrath S, Van Sinderen D (2007) Bacteriophage: Genetics and Molecular Biology (1st edn). Caister Academic Press, Uk.
- Beg MA, Thakur SC, Meena LS (2018) Structural prediction and mutational analysis of Rv3906c gene of Mycobacterium tuberculosis H37Rv to determine its essentiality in survival. Advances in bioinformatics Pp 1-12.
- Mankiewicz E (1961) Mycobacteriophages isolated from Persons with Tuberculous and Non-tuberculous Conditions. Nature 191: 1416-1417.
- Soloff BL Sol, Rado TA, Henry BE 2nd, Bates JH (1978) Biochemical and morphological characterization of Mycobacteriophage R1. J Virol 25(1): 253-262.
- Doss J, Culbertson K, Hahn D, Camacho J, Barekzi N (2017) A review of phage therapy against bacterial pathogens of aquatic and terrestrial organisms. Viruses 9(3): 50.
- Lwoff André (1953) Lysogeny. Bacteriol Rev 17(4): 269-337.
- Sassi M, Bebeacua C, Drancourt M, Cambillau C (2013) The first structure of a Mycobacteriophage, the Mycobacterium abscessus subsp. Bolletii phage Araucaria. J Virol 87(14): 8099-8109.
- Catalão MJ, Gil F, Moniz Pereira J, Pimentel M (2010) The Mycobacteriophage Ms6 encodes a chaperone‐like protein involved in the endolysin delivery to the peptidoglycan. Mol Microbiol 77(3): 672-686.
- Gil F, Catalão MJ, Moniz Pereira J, Leandro P, McNeil M, Pimentel M (2008) The lytic cassette of Mycobacteriophage Ms6 encodes an enzyme with lipolytic activity. Microbiology 154(5): 1364-1371.
- Daffé Mamadou, Philip Draper (1997) The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol 39: 131-203.
- Catalão MJ, Pimentel M (2018) Mycobacteriophage Lysis Enzymes: Targeting the Mycobacterial Cell Envelope. Viruses 10(8) Pp 428.
- Gil F, Grzegorzewicz AE, Catalão MJ, Vital J, McNeil MR, et al. (2010) Mycobacteriophage Ms6 LysB specifically targets the outer membrane of Mycobacterium smegmatis. Microbiology 156(5): 1497-1504.
- Danelishvili Lia, Lowell S Young, Luiz E Bermudez (2006) In vivo efficacy of phage therapy for Mycobacterium avium infection as delivered by a nonvirulent Mycobacterium. Microb Drug Resist 12(1): 1-6.
- Foley Thomas EM, Whipple DL, Bermudez LE, Barletta RG (1995) Phage infection, transfection and transformation of Mycobacterium avium complex and Mycobacterium paratuberculosis. Microbiology 141(5): 1173-1181.
- Broxmeyer L, Sosnowska D, Miltner E, Chacón O, Wagner D, et al. (2002) Killing of Mycobacterium avium and Mycobacterium tuberculosis by a Mycobacteriophage delivered by a nonvirulent Mycobacterium: A model for phage therapy of intracellular bacterial pathogens. J Infect Dis 186(8): 1155-1160.
- Puck Theodore T, Alan Garen, Jewell Cline (1951) The mechanism of virus attachment to host cells: I. The role of ions in the primary reaction. J Exp Med 93(1): 65-88.
- Satish Rajitha, Anita Desouza (2019) Study of characteristics of Mycobacteriophage–A novel tool to treat Mycobacterium spp. Int J Mycobacteriol 8(2): 170-174.
- Tokunaga Tohru, Margret I Sellers (1964) Infection of Mycobacterium smegmatis with D29 phage DNA. J Exp Med 119(1): 139-149.
- Sellers Margret I, Wm L Baxter, HR Runnals (1962)"Growth characteristics of Mycobacteriophages D28 and D29. Can J Microbiol 8: 389-399.
- McNerney R, Wilson SM, Sidhu AM, Harley VS, Al Suwaidi Z, et al. (1998) Inactivation of Mycobacteriophage D29 using ferrous ammonium sulphate as a tool for the detection of viable Mycobacterium smegmatis and tuberculosis. Res Microbiol 149(7): 487-495.
- Samaddar S, Grewal RK, Sinha S, Ghosh S, Roy S, et al. (2015) Dynamics of Mycobacteriophage-mycobacterial host interaction: evidence for secondary mechanisms for host lethality. Appl Environ Microbiol 82(1): 124-133.
- Monu, Meena LS (2016) Roles of Triolein and Lipolytic Protein in the Pathogenesis and Survival of Mycobacterium tuberculosis: A Novel Therapeutic Approach. Appl Biochem Biotechnol 178 (7): 1377-1389.
- Rajni, Meena LS (2011) Unique characteristic features of Mycobacterium tuberculosis in relation to Immune system. American Journal of Immunology 7 (1): 1-8.
- Shivangi, Beg MA, LS Meena (2019) Mutational effects on structural stability of SRP pathway dependent co-translational protein ftsY of Mycobacterium tuberculosis Gene Reports 15.
- Meena J, Meena LS (2015) Scope and perspectives of new TB drugs and vaccines. American Journal of Infectious Diseases 11 (3): 63-73.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...