Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Trace Element Analysis of Dental Powders by X-ray Fluorescence Technique Volume 2 - Issue 2
Daisy Joseph*
- Nuclear Physics Division, BARC, India
Received:August 20, 2019; Published:September 17, 2019
*Corresponding author:Daisy Joseph,Nuclear Physics Division, BARC, India
DOI: 10.32474/ANOAJ.2019.02.000131
Abstract
Dental powder has been analyzed using conventional XRF and handheld XRF (HHXRF). The spectrum shows a large amount of Si, K, Ca and Zr in XRF and Al in HHXRF. The HHXRF has an advantage of showing Al which is a prominent element in dental powders. To obtain Al which is present in dental powders HHXRF is the preferred technique to conventional XRF. The present work will highlight the experimental technique, elements expected in dental powder and conclusions therein.
Introduction
For more than 160 years dentistry has used silver amalgam, which contains approximately 50% Hg metal, as the preferred tooth filling material. During the past decade medical research has demonstrated that this Hg is continuously released as vapor into mouth air; then it is inhaled, absorbed into body tissues, oxidized to ionic Hg, and finally covalently bound to cell proteins [1-3]. The efficiency of dental filling materials when tested with radio-isotopes reveals that all fillings leak mouth fluids between the filling and the tooth. These studies were carried out to investigate the presence of toxic elements in dental powder. They can lead to the way to new discovery of better dental filling materials. Hence dental powders have been analyzed using conventional XRF and handheld XRF (HHXRF). The spectrum shows a large amount of Si, K, Ca and Zr in XRF and Al in HHXRF. The HHXRF has an advantage of showing Al which is a prominent element in dental powders. To obtain Al which is present in dental powders HHXRF is the preferred technique to conventional XRF. The present work will highlight the experimental technique, elements expected in dental powder and conclusions therein.
Experimental Methods
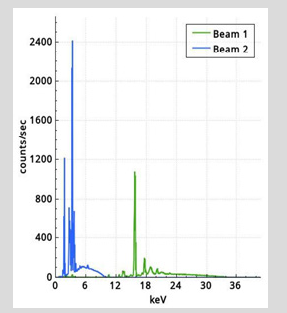
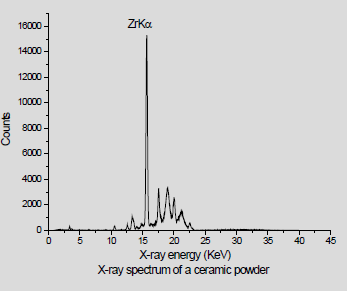
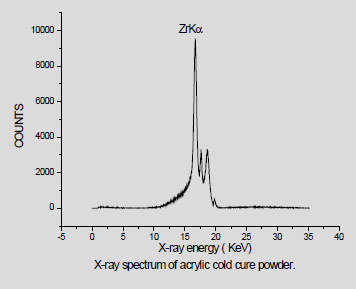
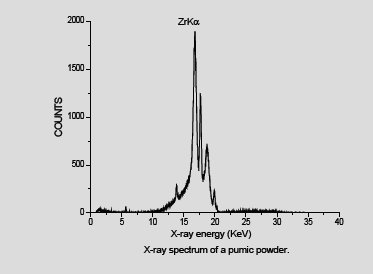
We have analyzed a no of dental powders which is Ceremic powder, Acrylic cold cure powder and Pumic powder using conventional XRF ( X-ray tube ) Dental porcelain (also known as dental ceramic) is a dental material used by dental technicians to create biocompatible lifelike dental restorations, such as crowns, bridges, and veneers (Figure 1). For EDXRF measurements a Jordan Valley EDXRF spectrometer (EX-3600 TEC) with a Rh target low power X-ray tube was used. The spectrometer is equipped with a ten-position sample chamber. For the detection of X-rays, a peltier cooled Si-PIN Diode detector with a resolution of 150eV at 5.9keV (Mn Kα) was used. For background reduction in the spectra Rh radiation filter was used. The applied voltage and current were 40kV, 1000μA, respectively. The samples were taken in a 12-micron thick mylar window sample holder and for the measurement of EDXRF spectra. The measurement time was 100s. Handheld XRF (HHXRF) is the need of the hour to analyze metals, powders and alloys. To test the efficiency of the HHXRF a dental powder which was used as a test sample, was analyzed to detect the trace elements. The dental powders were obtained from the dental section of BARC Hospital. The sample was powdered finely and was placed in a cubical box for irradiation by X-ray tube. (Rhodium tube) was used for irradiation. The spectrum was obtained in 60 seconds. A Typical spectrum of the dental powder is shown in Figure 2. The prominent peaks of K (3.3keV) and Zr (15.7 KeV) are seen. The beam lines were of (Beam 1 from 12 to 36keV) and Beam 2 from 0-12KeV) (Table 1). In the case of conventional XRF the figures are as follows Figure 3-6.
Results and Discussion
Figures 4-6 shows spectrum obtained from XRF. It is observed that they are devoid of Al as Al is absorbed in the detector window. However, Al is observed in the spectrum by HHXRF (Figure 2). All is an essential element in dental powders as dental powders has a composition of polycrystalline solids which is Al2O3. Al cannot be detected by many conventional instruments in XRF due to the detector window absorbing low energy X-rays, However Al can be detected by HHXRF. HHXRF has SDD (Silicon drift detector) which has a graphene window and the Al X-rays do not get absorbed unlike in the Si (Li) (Lithium drifted Silicon X-ray detector which has Be window and absorbs low energy X-rays below Z<19. The analysis shows that Si is the major element followed by K, Al, Ca, Zr and other minor elements like P, Ca, V, Cr, Fe, Cu, Rb, Sr, Y. It is observed that Si is present significantly. Si is an essential element in the bodily functions especially the bones, Si is essential for healing process and also for building the cellular structures, cytoskeleton. Ca is essential in bone building and Zr also in muscle control. It is observed that Hg is not present.
Conclusion
Al, Si, K, Ca and Zr are seen to be present significantly which are useful elements. There are no toxic elements which may harm the dental procedures. The role of each of these elements needs to be investigated and the study is underway for further conclusions. HHXRF can be a useful tool for detecting Al in samples
Acknowledgements
I acknowledge the services of I. R. Technology Services Pvt. Ltd., Mahape, for the analysis of the dental powder by HHXRF and also the services of Dr. NL Mishra and Shri Sanjay Kumar, FCD for their analysis of dental powders by conventional XRF.
References
- (1996) World Health Organization. Trace elements in human nutrition and health. ISBN: 92 4 1561734
- Trace elements. Wikipedia, the free encyclopedia.
- Nguta JM (2010) Essential trace elements: Trace elements in human and animal health., Lambert Academic Publishing, LAP, Germany, ISBN-NR: 978-3-8433-7174-2.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...