Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Nicardipine: Concise Review on Analytical Techniques Volume 1 - Issue 5
Pritam Jain S*, Vrushali Patil A, Ansari Shoaib Ahmed Ayaz Ahmed, Champalal Pawara T and Sanjay Surana J
- R.C. Patel Institute of Pharmaceutical Education and Research, India
Received:January 28, 2019; Published:February 14, 2019
*Corresponding author:Pritam Jain S, R.C. Patel Institute of Pharmaceutical Education and Research, India
DOI: 10.32474/ANOAJ.2019.01.000123
Abstract
Nicardipine (NCD) is the dihydropyridine class of calcium channel blockers. This study exits a concise review of analytical methods for the quantification of NCD in pharmaceutical preparations and biological fluids. NCD accessible single or in combination with added drugs in pharmaceutical matrices with many drugs like nifedipine, isradipine, veramipril, amlodipine and Aliskiren. They include numerous analytical techniques defined in this study for NCD were high-performance liquid chromatography (HPLC), UV-spectrophotometry, spectrofluorometry, electrochemical methods and liquid chromatography-mass spectroscopy (LCMS). The concise review explains the percentage utilization of the various approaches for analysis of NCD.
Keywords: Nicardipine; Review; Analytical method; Chromatography
Abbreviations:NCD: Nicardipine; NCD HCl: Nicardipine hydrochloride; AML: Amlodipine; NIF: Nifedipine; ISRA: Isradipine; VRP HCl: Verapamil hydrochloride; DTZ HCl: Diltiazem hydrochloride; FLN: Flunarizine; ALI: Aliskiren; MP: Metoprolol; TRB: Terbutaline; PRO: Propranolol; OXA- Oxazepam; FLUX: Fluoxetine; PIND: Pindolol; SDS: Sodium dodecyl sulphate; TEA: Triethylamine; LCMS: Liquid chromatography- mass spectroscopy; LC/MS/MS: Liquid chromatography- mass spectroscopy- mass spectroscopy; LC-ESI- MS: Liquid chromatography-electrospray- mass spectrometry; RSD: Relative standard deviations; ACC: Accuracy; PRECI: Precision; SS: System suitability; RF: Retention factor; RT: Retention time; TP: Theoretical plate; TF: Tailing factor; RES: Resolution; FR: Flow rate; nm: Nanometre; PDA: Photodiode array detector; SDM: Second derivative method; CAN: Acetonitrile; FA: Formic acid; GCE: Glassy carbon electrode; MPE: Mercury pool electrode; PE: Platinum electrode; CPE: Carbon paste electrode; PMDE: Paste mercury drop electrode; HMDE: Hanging mercury drop electrode
Introduction
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
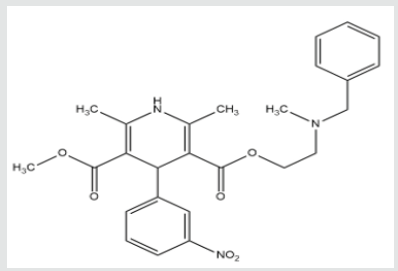
Nicardipine is chemically identified as 1, 4-Dihydro-2, 6-dimethyl-4-(3-nitrophenyl)-3, 5-pyridinedicarboxylic acid methyl 2-[methyl-(phenylmethyl) amino]ethyl ester (Figure 1). The molecular weight of NCD is 479.52 [1]. The melting point of NCD in the range of 136-138 °C (277-280 °F) [2].It is a calcium channel antagonist class of dihydropyridine. It is now used for the treatment of angina pectoris and similarly used in the managing of hypertension [3]. Most CCBs, including amlodipine, felodipine, isradipine, nicardipine, and nifedipine, belong to the dihydropyridine class [4]. The dihydropyridine have little direct effect on cardiac tissue at typical therapeutic levels; yet, they can evoke reflex tachycardia [5].
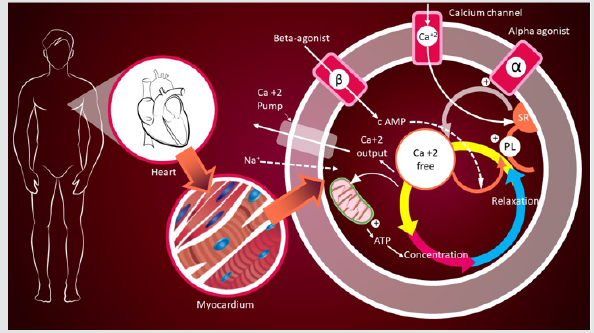
The schematic diagram shows the molecular mechanism of NCD (Figure 2). The CCBs indicated a number of important differences from the pharmacokinetic and pharmacodynamics point of views as well as for selectivity and duration of pharmacological action, while distribution the equal ability to interact with L-type voltagedependent transmembrane calcium channels [6,7].
Figure 2:Schematic representation to explain the molecular mechanisms of actions of calcium channel blockers (CCBs).
Chemistry of NCD
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
It is a synthetic derivative of potent calcium channel blocker and nitrophenyl-pyridine [8]. The IUPAC name of nicardipine is 5-O-[2-[benzyl (methyl) amino] ethyl] 3-O-methyl 2, 6-dimethyl- 4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate [9]. The molecular formula is C26H29N3O6 and molecular weight is 479.533 g/mol [10]. NCD clinical properties and molecular mechanisms closely similar to those of nifedipine and the other dihydropyridine but nicardipine is extra selective for coronary and cerebral blood vessels. As compare to nifedipine the nicardipine have longer halflife [11].
Pharmacokinetic Properties
Almost 95% of nicardipine is bound by serum proteins, precisely α1-acid glycoproteins (AAG), albumin, and lipoproteins [4]. Nicardipine plasma elimination half-life has reached between 44 to 107 minutes in maximum studies with clearance of the drug due mostly to hepatic mechanisms [12]. The orally directed nicardipine is speedily absorbed and peak plasma concentrations arising between 20 minutes and 2 hours [13]. Distribution volume in normal volunteers have reached between 0.6L/kg to 63L. Lower than 0.03% of parent drug is enhanced from the urine of humans [14].
Pharmacodynamics Properties
Oral doses and intravenous of nicardipine may create rises in heart rate of up to 30% and 8 to 26% and dose-related reductions in mean arterial blood pressure correspondingly, and the duration of these effects which may be as long as 3 hours have generally been greater in patients at rest than in those at exercise [15,16]. While decreases in blood pressure have been efficiently preserved for some months without indication of tachyphylaxis, increases in heart rate occasionally seen after acute administration are not detected later extensive time oral treatment [15].
Nicardipine is related to further peripheral vasodilators (Figure 3) [17]. Nicardipine constrains the influx of extra cellular calcium crosswise the myocardial and vascular smooth muscle cell membranes maybe by deforming the channel [18]. The reduction in intracellular calcium constrains the contractile developments of the myocardial smooth muscle cells, affecting dilation of the coronary and systemic arteries, enhanced oxygen transfer to the myocardial tissue, diminished total peripheral resistance, reduced systemic blood pressure, and decreased afterload [19].
Figure 3:The proposed in vitro metabolic pathways of nicardipine in human liver microsomes are illustrated in, [4].
Analytical Method
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
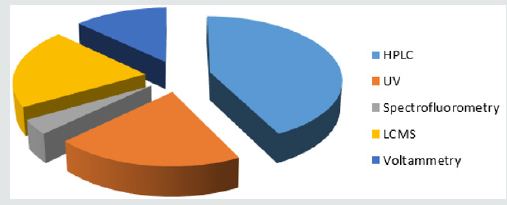
The substantial literature survey exposed that, the development and validation of analytical methods for identification and quantification of drugs and other molecules of interest in Pharmaceutical Matrices [20,21]. Various analytical methods were developed viz HPLC, UV/Visible spectrophotometry, spectrofluorometric, LC-MS and voltammetry for estimation of NCD the dosage form [22]. The proposed method represents the determination of NCD in single and simultaneous method with Nifedipine, Isradipine, Aliskiren, Amlodipine, Verapamil HCl, Diltiazem HCl and Flunarizine [23].
Bioanalytical Method
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
Validated, Choosy and complex analytical systems for the quantifiable estimation of drugs and their metabolites (analytes) and biomarkers are serious for the effective manner of nonclinical and biopharmaceutics and clinical pharmacology studies [24,25]. Validating bioanalytical techniques contains carrying out all of the processes that establish that a specific method used for quantifiable measurement of analytes in a certain biological medium (e.g., blood, plasma, urine, or serum) is reproducible and dependable for the proposed use [26].
Analytical Acounts on Nicardipine
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
HPLC
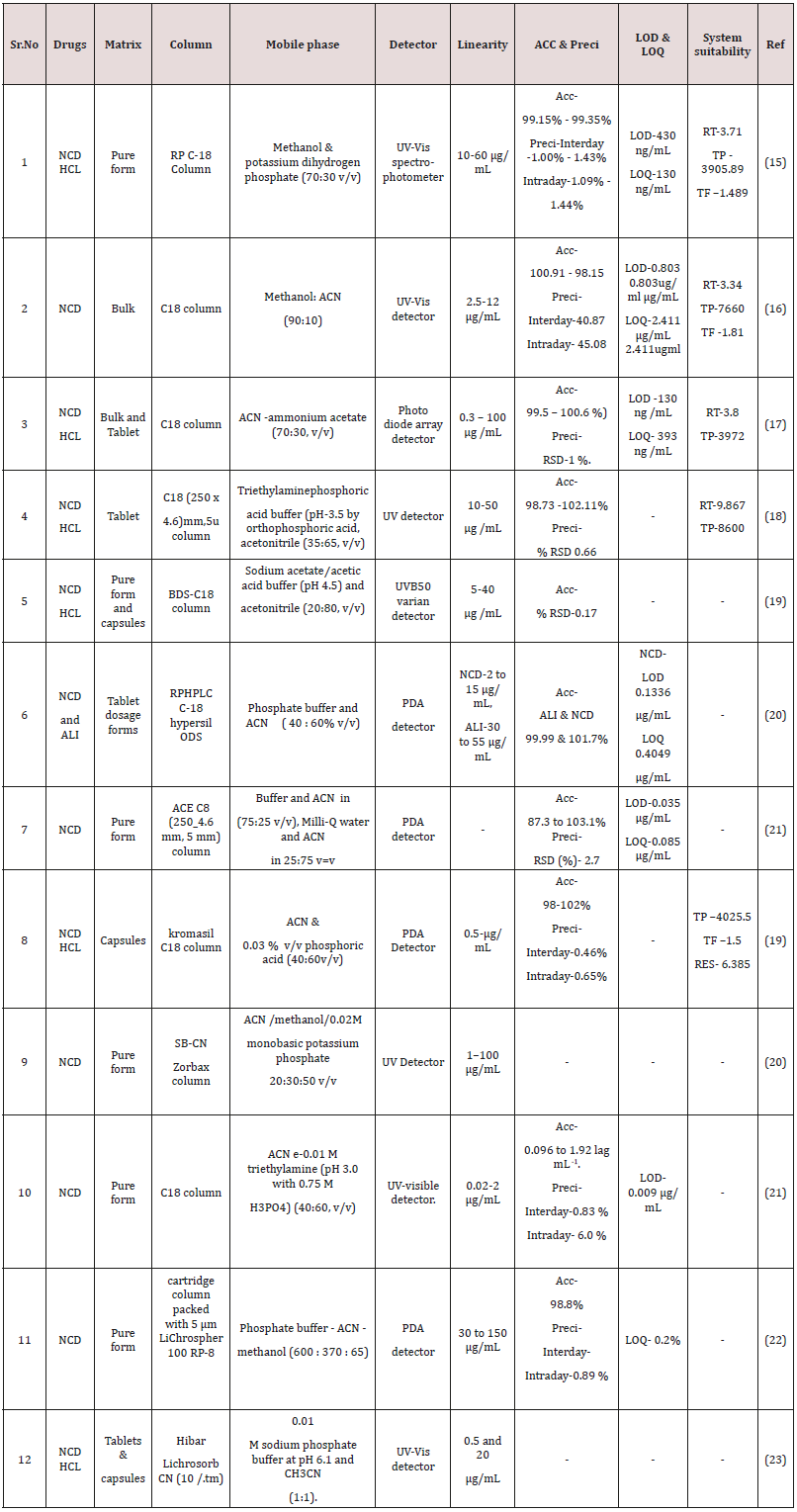
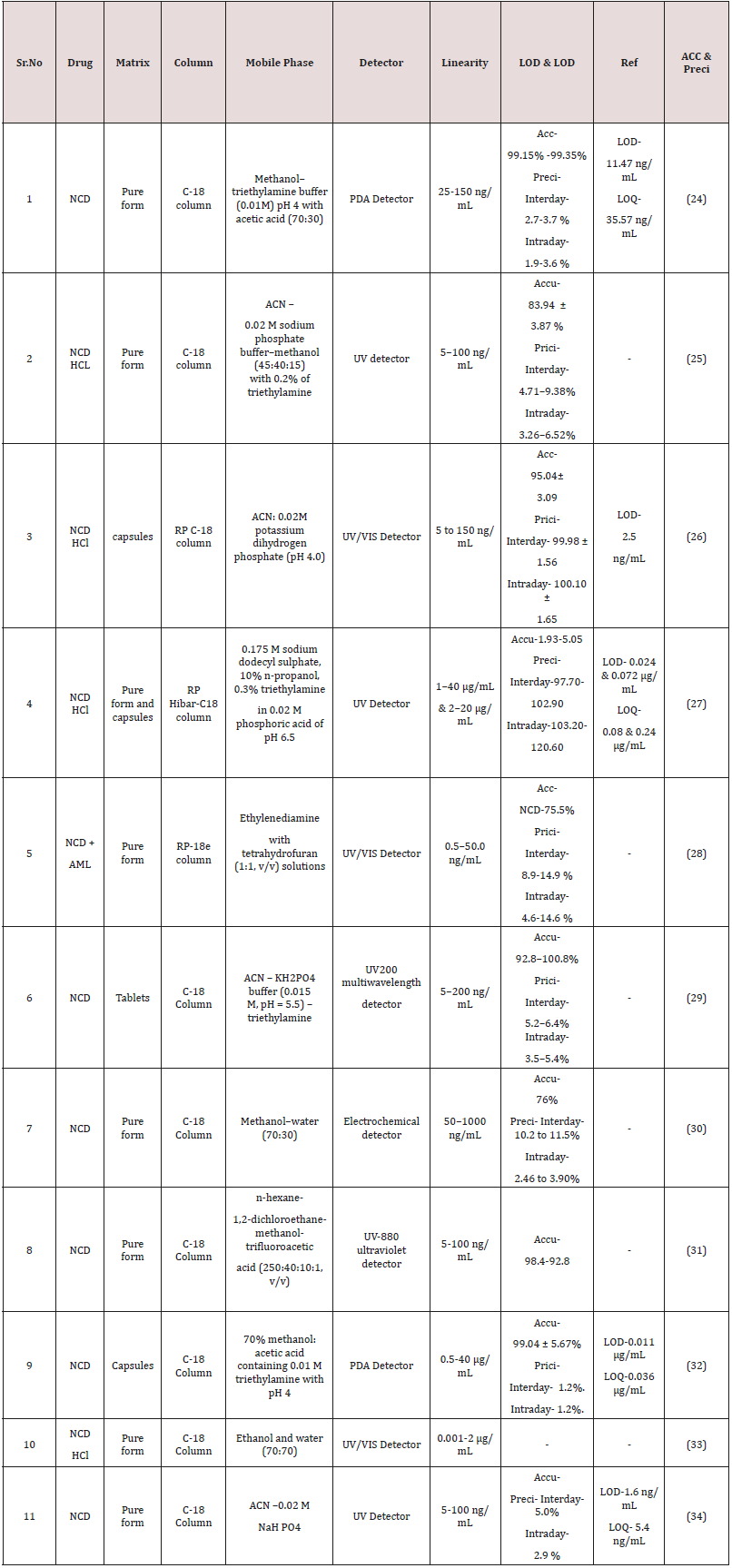
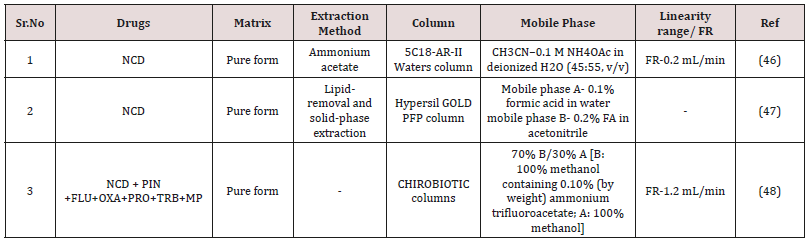
(Figure 4) The technique of high performance liquid chromatography (HPLC) was established in the late 1960s and early 1970s from knowledge of the theoretical principle that already had been recognized for the earlier chromatographic technique [27]. The method is created on the same modes of separation as classical column chromatographic, i.e. adsorption, partition (including reversed-phase partition), ion exchange and gel permeation, but it differs from column chromatography in that the mobile phase is pumped over the packed column underneath high pressure [28,29]. Estimation of NCD single or in combination with added drugs in pharmaceutical matrices and biological fluid by HPLC are note down in (Table 1 & 2) [30].
Analytical Determination of NCD
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
Tiwari R N et al. [31] initiated validation and Development of an analytical method for the RP- HPLC for nicardipine hydrochloride. Kromacil C-18 column on an isocratic mode was developed and separation was done by a mobile phase comprising methanol and potassium dihydrogen phosphate (70:30v/v) and pH of buffer scheme was used to 3.0 through ortho phosphoric acid. UV observation at 236nm and flow rate was retained 1ml/min. The LOD and LOQ were originate to be 430ng /mL and 130ng /mL. The linearity of the suggested method in the series of 10-60μg/ml, with a regression coefficient of 0.9994 and the % recovery was 99.15% to 99.35%.The established process can be used for the repetitive analysis and assay of nicardipine HCl in quality control laboratories.
Veena S. Kasture et al. [32] initiated a isocratic RP-HPLC method was developed and validated for Nicardipine hydrochloride in bulk and formulation.C18 column with dimension on 25 x 0.6cm and the mobile phase containing the of mixture of methanol: acetonitrile in proportion of (90:10). The UV observation was carried out at wavelength 354nm. The LOD and LOQ were originate to be 0.803μg/ml and 2.411μg/ml. The linearity of the suggested method in the series of 2-12μg/ml.
Jyoti Salvekar et al. [33] initiated stability indicating RP-HPLC method was established and formalize for nicardipine hydrochloride (NC) in the occurrence of its degradation products. The linearity of the suggested method in the series of 0.3-100μg/mL. C18 column with dimension 150mm length, 4.6mm ID and 5μm particle size was used for the method expansion and chromatographic separation was done by a mobile phase comprising a combination of aqueous 0.1M ammonium acetate and acetonitrile in the proportion (30:70, v/v). The flow rate and detection wavelength were 1.2mL min‐1 and 237nm. The LOD and LOQ were originate to be 130ng/ mL and 393ng/ mL.
Milind B. Ubale et al. [34] Established stability-indicating isocratic reversed phase high-performance liquid chromatographic method was established and validated for quantifiable determination of Nicardipine hydrochloride in bulk drugs. Method was developed using C18 (250 x4.6)mm, 5um column and separation was achieved by using a mobile phase used of triethylaminephosphoric acid buffer (pH-3.5 by orthophosphoric acid, acetonitrile (35:65,v/v). The method was linear in the range of 0.3-100μgmL‐1 nicardipine concentration [35]. The flow rate and detection wavelength were 1.0ml/min and 353nm.
Shahul Hameed M et al. [36] Established analytical RP-HPLC method for simultaneous estimation of Aliskiren Hemifumarate and Nicardipine Besylate from. Method was developed C-18 hypersil ODS column and separation was achieved by using a mobile phase containing phosphate buffer and acetonitrile ( 40 : 60% v/v). The method was linear in the range for Nicardipine 2 to 15μg/mL and Aliskiren 30 to 55μg/mL concentration. The flow rate and detection
wavelength kept at 1mL/min and at 237nm. The LOD and LOQ were found to be for Aliskiren Hemifumarate and Nicardipine Besylate 0.1614μg/mL and 0.1336μg/mL and 0.4890μg/mL and 0.4049μg/ mL. . The percent recovery for Aliskiren and Nicardipine in ranged 99.99 and 101.7% and correlation coefficient (R2) were 0.9990 [36].Bioanalytical determination of NCD
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
Sheikha M. Al-Ghannam et al. [37] Initiated reversed-phase liquid chromatography method was established for the purpose of nicardipine hydrochloride in human plasma. Nicardipine were initially extracted from hexane-butanol (12:1v/v). Nicardipine was separated by HPLC C-18 column and quantified by ultraviolet detection at 353nm. A mixture of methanol/TEA buffer (0.01M) pH 4 with acetic acid (70:30v/v) was used as mobile phase. The linearity of the proposed method in the range of 15-200μg/ml, with a regression coefficient of 0.9991 and the % recovery was 99.15% to 99.35%. The LOD and LOQ were originate to be in plasma 11.74 and 35.57ng/mL. The RSD of intra- and inter-day examination for NCD in plasma were 2.7-3.7% and 1.9-3.6% [37].
P. Bhaskar et al. [38] Established high-performance liquid chromatographic method for the nicardipine hydrochloride in human plasma. RP C-18 was used for separation with mobile phase comprising acetonitrile: 0.02M potassium dihydrogen phosphate (pH 4.0) in the proportion of 60:40v/v. Ultraviolet detection was conceded at 239nm. Extracted with ethyl acetate. The method proved to be linear in the choice of 5-150ng/0.5 mL of plasma with a regression coefficient (r2) of 0.9987. The run time was fixed at 10 minutes. The range of percentage of RSD for intra-day analyses inter-day analyses was smaller than 2.5% and limit of detection 2.5ng/0.5mL in plasma.
M. I. Walash et al. [39] Initiated a isocratic reversed phase stability-indicating HPLC technique was established and validated for quantifiable purpose of nicardipine hydrochloride. Nicardipine was separated by a Hibar-C18 (150 _ 4.6mm i.d.) stainless steel column. A mixture of was used as mobile phase 10% n-propanol, 0.175 M SDS, 0.3% TEA in 0.02 M phosphoric acid of pH 6.5. The method was linear for 1-40 and correlation coefficient (R2) were 0.9999.The flow rate were 1 ml/min. The LOD and LOQ were found to be 0.024μg/ml and 0.08μg/ml. The mean % recovery 100.12+0.28 and 100.87+0.41, respectively.
R.M. Alonso et al. [40] They performed high-performance liquid chromatographic technique using electrochemical detection for the estimation of six 1,4-dihydropyridines. Method was established by means of a Supelcosil LC ABZ1Plus C column and mobile phase comprising methanol-18 water (70:30), containing 2mM CH COOHCH COONa. The intra-day difference was originate to be smaller than 5.0%. The flow rate were 1ml /min. The method was linear for 50-1000ng/mL and correlation coefficient (R2) were 0.9998. The relative standard deviations of intra -day and inter-day analysis for NC 2.46-3.90 and 10.2-11.5% in plasma. The relative standard devation were create to be 4.6% .
Sheikha M. Al-Ghannam et al. [41] They performed stability indicating RP-HPLC technique for the purpose of nicardipine (NC) in the presence of its degradation products. They used C18 (150mm, 3.9mm, 5lm) analytical column with UV observation at 353nm. A mixture of 70% methanol: acetic acid comprising 0.01M triethylamine with pH 4 was handed-down as the mobile phase with flow rate of 1.0mL/min. The calibration curve is linear over the concentration range 0.5-40μg/mL with R2 was originated to be 0.9991. The LOD and LOQ were found to be 0.011μg mL‐1 and 0.036μg /mL, respectively. The mean % recoveries of 100.11± 2.26%, respectively.
Spectrophotometry Method
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
Inside the literature about 9 methods were designated for the assessment of NCD using spectrophotometry, of which 6 methods are for estimation NCD alone, while the others NCD in combination with other drug substances. In the literature also, 2 spectrofluorometric methods have been established of NCD in tablets [42,43]. Spectrophotometric and spectrofluorometric methods have been presented for the determination of NCD alone and in Combinations are note down in (Table 3) [44].
Estimation of NCD as single entity
Hayam Mahmoud Lotfy et al. [45] A confirmed humble and discriminating spectrophotometric method was established for simultaneous purpose of nicardipine in presence of its alkaline induced degradation products. The curve is linear in the choice of 2-18μg/mL. They dignified first derivative (D1) spectra and the second derivative (D2) at 382.3 and 239nm. The ratio derivative measured by the largeness at 244nm.
Amala Mateti et al. [46] They performed specific and correct technique has been developed and subsequently validated for estimation of Nicardipine hydrochloride. The linearity of the suggested method in the series of 5-25μg/ml, with R2 created to be 0.999. Acetonitrile: water (50:50) solvent were used and absorbtion maxima were 235 nm. The process found to be exact and precised. The LOD and LOQ were originate to be 0.3130μg/ml and 0.1032μg/ml.
Estimation of NCD in Combinations
S. M. AL-Ghannam et al. [47] Developed simple spectrophotometric technique was established for the purpose of 1,4-dihydropyridine composites for simultaneous determination of NCD and ISRA either in unpolluted form. Absorption maximum for red product were at 546 and 539nm with NCD and ISRA, respectively. The method were linear in series of 8.0-180μg/ml with the LOD of 1.67μg/ml for NCD and 8.0-110μg/ml with the LOD of 1.748μg/ml for ISRA.
Sayed M. Derayea et al. [48] They performed spectrophotometric technique was designated for purpose of AML and NCD in bulk precipitates and pharmaceutical preparation. The absorption maxima were at 549nm. The process linear in the series of 5-60μg/ ml for both drugs i.e. AML and NCD and correlation coefficients for amlodipine and nicardipine were (0.9981 and 0.9995). The LOD and LOQ were found to be 1.8 and 1.2μg/ml and 6.0 and 3.6μg/ml for both drugs. The mean percentage recoveries were created to be 100.04 ± 0.83 and 99.98 ± 0.80.
Ahmed A. H. Abdellatif et al. [49] They was described spectrophotometric technique for purpose of NIF and NIC in their pharmaceutical preparations. The method originated in series of range 2.0 to 12.0μg/mL with quantitation limit 1.4 and 1.9μg/mL. The mean percentage recoveries were established to be 98.2±0.3 to 99.5±0.3% NIF and NIC, individuallyd.
Spectrofluorometric Methods
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
Sheikha M. Al-Ghannam et al. [50] They implanted spectrofluorometric process for estimation 1,4-dihydropyridine compounds in pharmaceutical preparations and organic fluids. The nicardipine, nifedipine and isradipine were rectilinear above the series of 0.4- 6.0, 0.2-4.0 and 0.1-9.0μgml-1 and detection limit found to be 0.0028, 0.017 and 0.016μgml-1, correspondingly.
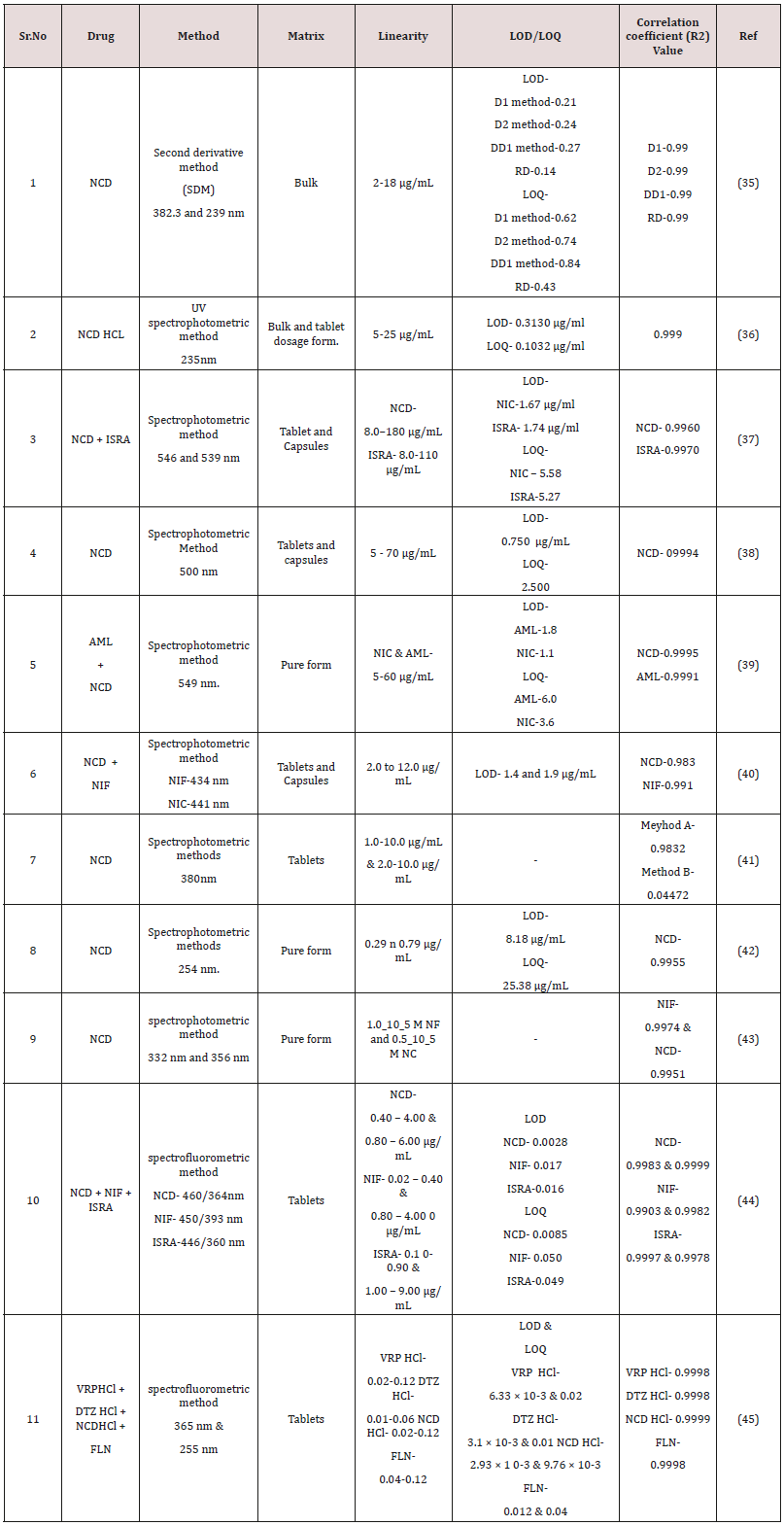
M. I. Walash et al. [51] They implanted sensitive spectrofluorometric method for estimation calcium channel blockers namely, verapamil hydrochloride, diltiazem hydrochloride, nicardipine hydrochloride and flunarizine. The fluorescence intensity-concentration plots for all compounds were linear over the ranges of 0.01 to 0.12 μgmL-1. The LOD for all compounds in ranged from 2.93 × 10-3 to 0.012μg mL-1 and LOQ from 9.76 × 10-3 to 0.04μg mL-1 were produced (Table 4).
Table 4: Spectrophotometric and Spectrofluorimetric methods for estimation of NCD alone and in combined dosage form.
Liquid Chromatography-Mass Spectrometric Methods
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
Analytical Determination of NCD
An-Bang Wu et al. [52] Reported sensitive and simple liquid chromatography/electrospray mass spectrometry (LC-ESI-MS) method for determination of nicardipine. Nicardipine sample of 0.104M in methanol was exposed to a Philips 400WUV lamp under normal atmosphere. In a photochemical chamber, the sample was exposed to irradiation for 3hr, by analyzing the HPLC chromatogram. Chromatographically separation was achieved on Inertsil 5C18-ARII Waters column (150 × 2.0mm i.d.) with CH3CN-0.1M NH4OAc in deionized H2O (45:55v/v) as the mobile phase. UV recognition at 254nm. The flow rate were 0.2mL/min with injection volume 2μL [52].
Shin-ichi Kubo et al. [53] They developed simple extraction technique for utilize both GC-MS and LC-MS/MS using the same extracted sample (Table 5). Chromatographically separation was reached on Hypersil GOLD PFP column with mobile phase A- 0.1% formic acid in water mobile phase B- 0.2% formic acid in acetonitrile.
Bioanalytical Determination of NCD
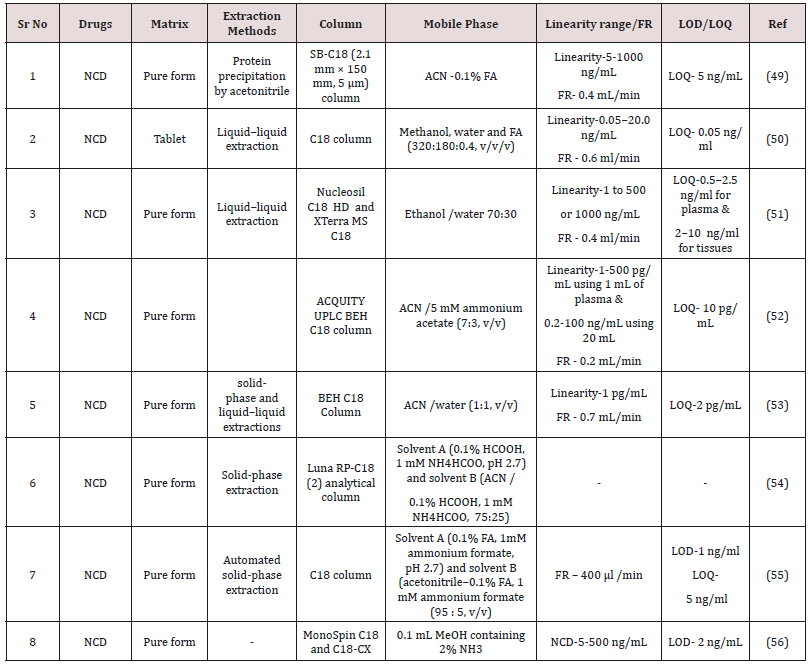
Mingjie DENG et al. [54] Reported sensitive liquid chromatography/electrospray mass spectrometry (LC-ESI-MS) technique for the valuation of nicardipine nicardipine in rat plasma. Midazolam handed-down as interior standard, were initially extracted from plasma by a protein precipitation by acetonitrile. Method was established using a C18 column and separation was reached by a mobile phase containing acetonitrile-0.1% formic acid with gradient elution. The method was linear 5-1000ng/mL for nicardipine in rat plasma and correlation coefficient (R2) were 0.996407. The flow rate were 0.4mL/min. The LOQ were found to 5ng/ml (Table 6). The intra- and inter-day variation was found to be less than 13%.
Meiling Qi et al. [55] They reported LC-MS technique has been settled and validated for the analysis of nicardipine in human plasma. Method was established using a SB-C18 (2.1mm ×150mm, 5μm) column and mobile phase containing methanol, water and formic acid (320:180:0.4,v/v/v). The process was linear in series of 0.05-20.0 ng/ml for nicardipine. The relative standard deviations of inter -day and intra-day analysis for NC ≤9.3 and 11.1%, Respectively. The mean recovery of nicardipine ± 4.9%.
Katja Heinig et al. [56] Repoeted LC-MS-MS methods can be used successfully for the determination of a wide variety of pharmaceutical compounds in plasma and tissues. They performed analytical assay for a variety of substances including nicardipine, nitrendipine, felodipine and benzodiazepines. Method was developed using a Nucleosil C18 HD and XTerra MS C18 Column and separation was achieved by using a mobile phase containing Ethanol /water 70:30. Accuracy and precision were originated to be in the series of 84.4-119.1% and 1-16.5%, respectively. LOD were originated to be in the seriese of 0.5-2.5ng/ml for plasma and 2-10ng/ml for tissues.
Claudia A. Mueller et al. [57] Reported simple and selective liquid chromatography-mass spectrometry (LC/MS/MS) screening method is described for the screening of 11 calcium channel blockers of the 1,4-dihydropyridine type in human plasma. Chromatographic separation of the analyte was achieved on a a reversed-phase C18 column, gradient elution using a mobile phase of solvent A 0.1% formic acid, 1mM ammonium formate, pH 2.7 and solvent B acetonitrile-0.1% formic acid, 1mM ammonium formate (95:5 v/v).
Voltammetric Methods
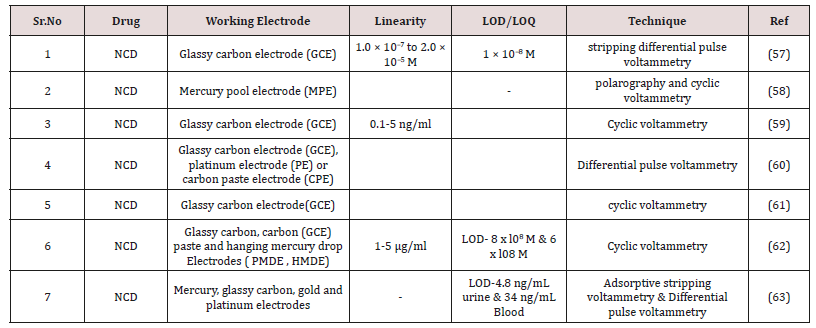
K. Zarei et al. [58] Implemented Stripping Voltammetric Purpose of Nicardipine Using β_Cyclodextrin Incorporated Carbon Nanotube. Modified Glassy Carbon Electrode. The method is linear above the series of 1.0 × 10-7 to 2.0 × 10-5M with correlation coefficient R2 = 0.9982. The LOD were calculated 1 × 10-8M. The suggested technique was efficaciously regisred to the purpose of nicardipine more to the blood serum.
A.El. Jammal et al. [59] Implemented electrochemical behavior of several calcium antagonists of the dihydropyridine class by using a glassy carbon electrode. Both oxidation of the dihydropyridine ring and reduction of the nitro group have been pointed out using cyclic voltammetry. Splitting of the nitro group reduction peak occurs when the dihydropyridine ring is first oxidized. On the other hand, the reduction pathway of the nitro group depends on its position.
Joseph Wang et al. [60] Implemented cyclic voltametric performance of nicardipine using a carbon paste, glassy carbon and hanging mercury drop electrodes. Nlcardipine contains two redox centers , a reducible aromatjc nitro group and an oxidizable dihydropyridine ring (Table 7). The method is linear above the series of 1-5μg/ml. The
Conclusion
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
The detailed review of the study high lights the current development of the analytical methods available for the quantification of NCD in bulk and pharmaceutical formulations and analysis of NCD in different matrices (such as plasma, serum, urine).A various investigation had perform including, HPLC, UV/ Vis-Spectroscopy, Spectrofluorometry, LC-MS and electrochemical method. and a greater work of methods by high-performance liquid chromatography and spectrophotometry were detected. The stability indicating assays have been developed for number of methods in the literature. The hyphenated LS-MS, LS-MS/MS method are reported for determination of VAL and its metabolite in plasma and other biological solutions. The aim of this article is to provide simple to use approaches with a correct scientific background to improve the quality of the analytical method development and validation process.
Acknowledgments
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
The authors wish to thank R.C. Patel Institute of Pharmaceutical Education and Research Shirpur, Dist. Dhule (MS) 425 405 for providing necessary library facilities.
References
- Abstract
- Introduction
- Chemistry of NCD
- Analytical Method
- Bioanalytical Method
- Analytical Acounts on Nicardipine
- Analytical Determination of NCD
- Bioanalytical determination of NCD
- Spectrophotometry Method
- Spectrofluorometric Methods
- Liquid Chromatography-Mass Spectrometric Methods
- Conclusion
- Acknowledgments
- References
- The Merck Index an Encyclopedia of chemical, drugs and biological (14th edn), pp. 6495.
- https://en.wikipedia.org/wiki/Nicardipine.
- Lotfy HM, Hegazy MAEM, El-Aziz A, Mai M, Fattah A, Elsayed L (2016) Stability Indicating Spectrophotometric Methods For Determination Of Nicardipine In The Presence Of Its Alkaline Induced Degradation Products.
- Nakamura K, Ariyoshi N, Iwatsubo T, Fukunaga Y, Higuchi S, et al. (2005) Inhibitory effects of nicardipine to cytochrome P450 (CYP) in human liver microsomes. Biological and Pharmaceutical Bulletin 28(5): 882- 885.
- George Brenner M, Craig Stevens W (2010) Text Book of pharmacology (3rd edn). Published by an arrangement with Elsevier Inc.
- https://www.drugs.com/pro/nicardipine.html
- https://www.researchgate.net/publication/268336319.
- Ibrahim KE, Al-Ashban RM, Babiker LB (2010) A Selective High Performance Liquid chromatographic Method to follow the hydrolytic degradation of Nicardipine hydrochloride. Journal of Chemistry 7(1): 85-92.
- Vaghela BK, Rao SS (2013) Identification and characterization of a novel potential degradant and development and validation of stabilityindicating rp-lc method for nicardipine impurities in injectable dosage form. Journal of Liquid Chromatography & Related Technologies 36(15): 2166-2181.
- https://www.researchgate.net/publication/12180525.
- Sorkin EM, Clissold SP (1987) Nicardipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy, in the treatment of angina pectoris, hypertension and related cardiovascular disorders. Drugs 33(4): 296-345.
- Yaswanth Kumar D, Arvind B karadi, Sandhya D, Appala Raju N (2012) Stability Indicating RP-LC Method for the Quantitation of Nicardipine Hydrochloride in Capsules. Journal of Pharmacy Research 5(5): 2473- 2476.
- Aboofazeli R, Zia H, Needham TE (2002) Transdermal delivery of nicardipine: an approach to in vitro permeation enhancement. Drug Delivery 9(4): 239-247.
- https://www.drugs.com/pro/nicardipine.html
- Fernandes CM, Veiga FJB (2003) A simple method for nicardipine hydrochloride quantification in plasma using solid‐phase extraction and reversed‐phase high‐performance liquid chromatography. Biomedical Chromatography 17(1): 33-38.
- Wei X, Yang G, Qi L, Chen Y (2009) Determination of nicardipine and amlodipine in human plasma using on-line solid-phase extraction with a monolithic weak cation-exchange column. Talanta 77(3): 1197-1202.
- Li K, Zhang X, Yuan YS, Zhao FL (1998) A high‐performance liquid chromatographic method for the determination of nicardipine in plasma and its application to pharmacokinetics in humans. Biomedical Chromatography 12(6): 326-329.
- Uno T, Ohkubo T, Sugawara K (1997) Enantioselective high-performance liquid chromatographic determination of nicardipine in human plasma. Journal of Chromatography B Biomedical Sciences and Applications 698(1-2): 181-186.
- US Department of Health and Human Services (2001) Guidance for industry, bioanalytical method validation.
- Krishnaiah YS, Satyanarayana V, Karthikeyan RS (2002) Effect of Solvent System on the In Vitro permeability of Nicrdipine Hydrochloride throught Excised Rat Epidermis. J Pharm Pharmaceut Sci 5(2): 124-130.
- Meng QC, Cheung AT, Guvakov D, Weiss SJ, Savino JS, et al. (1998) Extraction and quantification of nicardipine in human plasma. Journal of Chromatography B Biomedical Sciences and Applications 718(1): 121- 127.
- El Hamd MA, Derayea SM, Abdelmageed OH, Askal HF (2013) A novel spectrophotometric method for determination of five 1, 4-dihydropyridine drugs in their tablets and capsules using vanillin reagent. American Journal of Analytical Chemistry 4(03): 148.
- Suneetha Y, Naidu NVS Sensitive Spectrophotometric Methods for the Determination of Nicardipine in Pharamaceuticals Using Bromothymol Blue and Methyl.
- Marciniec B, Ogrodowczyk M (2006) Thermal stability of 1, 4-dihydropyridine derivatives in solid state. Acta Pol Pharm 63: 477- 484.
- Yáñez C, Núñez‐Vergara LJ, Squella JA (2003) Differential Pulse Polarographic and UV‐Vis Spectrophotometric Study of Inclusion Complexes Formed by 1, 4‐Dihydropyridine Calcium Antagonists, Nifedipine and Nicardipine with β‐Cyclodextrin. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis 15(22): 1771-1777.
- Bakhtiar R, Tse FL (2000) High‐throughput chiral liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry 14(13): 1128-1135.
- Yamane N, Takami T, Tozuka Z, Sugiyama Y, Yamazaki A, et al. (2009) Microdose clinical trial: quantitative determination of nicardipine and prediction of metabolites in human plasma. Drug metabolism and pharmacokinetics 24(4): 389-403.
- Beckett AH, Stenlake JB Book of practical pharmaceutical chemistry (4th edn). part two, pp: 157.
- Togashi K, Mutaguchi K, Komuro S, Kataoka M, Yamazaki H, et al. (2016) Systematic approach to optimize a pretreatment method for ultrasensitive liquid chromatography with tandem mass spectrometry analysis of multiple target compounds in biological samples. Journal of separation science 39(16): 3212-3220.
- Baranda AB, Alonso RM, Jiménez RM, Weinmann, W (2006) Instability of calcium channel antagonists during sample preparation for LC–MS–MS analysis of serum samples. Forensic science international 156(1): 23-34.
- Kharad SL, Tiwari RN (2011) Development and validation of HPLC method for Nicardipine hydrochloride. Journal of Pharmacy Research 4(7): 2226-2227.
- Kasture VS, Pawar SS, Musmade DS, Patil PP, Gaware SR Synthesis, characterization and development of validated rp-hplc method for the estimation of process-related impurities innicardipine formulation.
- Gadkari T, Chandrachood PRANAV, Torane RASIKA, Tele SHAHAJI, Deshpande NIRMALA, et al. (2010) Forced degradation study to develop & validate stability indicating RP-LC method for quantification of nicardipine HCl in bulk and tablet formulation. Int J Pharm Pharm Sci 2: 162-164.
- Ubale MB, Dhakane VD, Chaudhari VR (2011) A validated stabilityindicating HPLC assay method for Nicardipine Hydrochloride in bulk drug and dosage form. Elixir Appl Chem 41: 5867-5870.
- Namera A, Saito T, Seki Y, Mizutani T, Murata K, et al. (2018) Highthroughput monospin extraction for quantification of cardiovascular drugs in serum coupled to high-performance liquid chromatography– mass spectrometry. Acta Chromatographica, pp 1-5.
- Hameed S, Jat RK, Indulatha VN (2017) Validation of hplc and uv visible methods for few selected blood pressure lowering drugs and their formulations. Universal Journal of Pharmaceutical Research.
- Al-Ghannam SM, Al-Olyan AM (2009) High-performance liquid chromatographic method for the determination of nicardipine in pure, pharmaceutical preparations and plasma and its application to pharmacokinetics in humans. Orbital: The Electronic Journal of Chemistry 1(1): 64-74.
- Krishnaiah YSR, Satyanarayana V, Bhaskar P (2004) High Performace Liquid Chromtographic Determination of Nicardipine Hydrochloride in Human Plasma. Journal of Chemistry 1(1): 38-42.
- Walash MI, Belal F, El‐Enany N, Abdelal A (2007) Microemulsion liquid chromatographic determination of nicardipine hydrochloride in pharmaceutical preparations and biological fluids. Application to stability studies. Journal of liquid chromatography & related technologies 30(8): 1015-1034.
- Lopez JA, Martınez V, Alonso RM, Jimenez RM (2000) High-performance liquid chromatography with amperometric detection applied to the screening of 1, 4-dihydropyridines in human plasma. Journal of Chromatography A 870(1-2): 105-114.
- Al-Ghannam SM, Al-Olayan AM (2014) Stability-indicating HPLC method for the determination of nicardipine in capsules and spiked human plasma. Identification of degradation products using HPLC/MS. Arabian Journal of Chemistry.
- Bollo S, Núñez‐Vergara LJ, Carbajo J, Squella JA (2000) Electroreduction of Nitroaryl‐1, 4‐dihydropyridines on a Mercury Pool Electrode in Mixed Media Analysis of the Reaction Products and Their Reactivity with Biomolecules. Journal of The Electrochemical Society 147(9): 3406- 3413.
- Alvarez-Lueje A, Nuñez‐Vergara LJ, Squella JA (1994) Voltammetric behavior of 1, 4‐dihydropyridine calcium antagonists. Electroanalysis 6(3): 259-264.
- Obendorf D, Stubauer, G (1995) Adsorptive stripping voltammetry of nicardipine at a HMDE; determination of trace levels nicardipine in blood and urine. Journal of pharmaceutical and biomedical analysis 13(11): 1339-1348.
- Lotfy HM, Hegazy MAEM, El-Aziz A, Mai M, Fattah A, et al. (2016) Stability Indicating Spectrophotometric Methods For Determination Of Nicardipine In The Presence Of Its Alkaline Induced Degradation Products.
- Amala Mateti, Kiran Aarelly, Manish Kumar Thimmaraju, Raghunandan N (2012) Method development and validation of nicardipine hydrochloride in bulk and formulation using UV spectrophotometric method. Journal of Chemical and Pharmaceutical Research 4(7): 3688-3694.
- Al-Ghannam SM, Al-Olyan AM (2009) Spectrophotometric determination of nicradipine and isradipine in pharmaceutical formulations. Chemical Industry and Chemical Engineering Quarterly 15(2): 69-76.
- Derayea SM, Askal HF, Abdel-Megeed OH, El Hamd MA (2012) Spectrophotometric determination of amlodipine and nicardipine in pharmaceutical formulations via binary complex formation with eosin Y. Journal of Applied Pharmaceutical Science 2(06): 84-89
- El Hamd MA, Abdellatif AAH, Derayea SM, Abdelmageed OH, Askal HF (2015) Spectrophotometric Determination of Nifedipine and Nicardipine in their Pharmaceutical Preparations. Industrial Chemistry.
- Al-Ghannam S, Al-Olyan, A (2008) Spectrofluorometric determination of nicardipine, nifedipine and isradipine in pharmaceutical preparations and biological fluids. Open Chemistry 6(2): 222-228.
- Walash MI, Belal F, El-Enany N, Abdelal AA (2009) Kinetic Spectrofluorometric determination of certain calcium channel blockers via oxidation with cerium (IV) in pharmaceutical preparations. International journal of biomedical science: IJBS 5(2): 146.
- Chen SM, Hsieh MC, Chao SH, Chang EE, Wang PY, et al. (2008) Separation and structure determination of nicardipine photoproducts by LC‐ESIMS. Biomedical Chromatography 22(9): 1008-1012.
- Hara K, Waters B, Ikematsu N, Tokuyasu T, Fujii H, et al. (2016) Development of a preparation method to produce a single sample that can be applied to both LC–MS/MS and GC–MS for the screening of postmortem specimens. Legal Medicine 21: 85-92.
- Deng M, Zhang Q, Zheng Y (2013) Quantification of Nicardipine in Rat Plasma by Liquid Chromatography/Electrospray Mass Spectrometry and its Application. Latin American Journal of Pharmacy 32(5): 774-778.
- Qi M, Wang P, Jin X (2006) Liquid chromatography–mass spectrometry method for the determination of nicardipine in human plasma. Journal of Chromatography B 830(1): 81-85.
- Heinig K, Bucheli F (2002) Application of column-switching liquid chromatography-tandem mass spectrometry for the determination of pharmaceutical compounds in tissue samples. Journal of Chromatography B 769(1): 9-26.
- Mueller CA, González AB, Weinmann W (2004) Screening for dihydropyridine calcium channel blockers in plasma by automated solid‐phase extraction and liquid chromatography/tandem mass spectrometry. Journal of mass spectrometry 39(6): 639-646.
- Zarei K, Fatemi L, Kor K (2015) Stripping voltammetric determination of nicardipine using β-cyclodextrin incorporated carbon nanotubemodified glassy carbon electrode. Journal of Analytical Chemistry 70(5): 615-620.
- El Jammal A, Vire JC, Patriarche GJ, Palmeiro ON (1992) Cyclic voltammetric study of some calcium antagonist dihydropyridines in aqueous medium. Electroanalysis 4(1): 57-64
- Wang J, Deshmukh BK, Bonakdar M (1985) Electrochemical behavior and determination of nicardipine. Analytical letters 18(9): 1087-1102.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...