Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Bactericidal Biodegradable Films with Silver Nanoparticles for the Treatment of Burns Volume 1 - Issue 4
Yunusov Khaydar Ergashovich*, Sarymsakov Abdushkur Abdukhalilovich and Rashidova Sayora Sharaphovna
- Institute of Polymer Chemistry and Physics, Academy of Sciences of the Republic of Uzbekistan
Received: July 11, 2018; Published: July 17, 2018
*Corresponding author: Yunusov Khaydar Ergashovich, Institute of Polymer Chemistry and Physics, Academy of Sciences of the Republic of Uzbekistan, 100128, Tashkent, Abdulla Kadyri, Uzbekistan
DOI: 10.32474/ANOAJ.2018.01.000118
Annotation
Silver nanoparticles stabilized in a solution of sodium carboxymethylcellulose (Na-CMC) with a degree of substitution of 0.65- 0.85 and degree of polymerization of 200-600 were synthesized. Physical, chemical properties, and antimicrobial activity of films prepared from sodium carboxymethylcellulose solutions containing silver nanoparticles were studied. The shapes, quantities, and sizes of the silver nanoparticles occurring in the sodium carboxymethylcellulose films were determined by atomic force microscopy and UV - spectroscopy. The shape, size, and quantity of silver nanoparticles determine their biological activity. At increase of the silver nanoparticle sizes of 5 up to 25 nm was found to enhance the microbicidal activitiy of the carboxymethylcellulose films.
Keywords: Silver, Nanoparticles, Sodium Carboxymethylcellulose, Degree of Substitution (Ds), Degree of Polymerization (DP)
Introduction
Design of medicinal biodegradable polymer films containing silver nanoparticles is one of promising ways in the development of new chemical and pharmaceutical products. Such films exhibit prolonged therapeutic and bactericidal activity [1]. The bactericidal properties of silver and its derivatives are well-known since ancient times. They are used in medicine. Recently, reports on the correlation between bactericidal properties of silver and its particle size have been published. In addition, it was shown that a decrease of the sizes of silver nanoparticles lead to a proportional increase in their antibacterial activity [2]. The synthesis of silver nanoparticles with a specified shape and size, stable and capable to keep their high chemical and biological activity for a long time is an important task [3]. Silver nanoparticles inhibit the activity of the enzymeproviding oxygen exchange in microorganisms, such as pathogenic bacteria, viruses, and fungi (about 700 species of pathogenic flora and fauna) [4]. The transition from the ionic Ag+ form to metallic nanoclusters makes possible to reduce silver toxicity to cells of higher organisms without suppression of the antimicrobial activity against pathogenic microflora. Silver nanoparticles, especially stabilized ones, have greater stability and prolonged action. Sodium carboxymethylcellulose (Na-CMC), water - soluble film forming biodegradable polymer is widely used in the production of oral pharmaceuticals and drugs for external use primarily to increase the viscosities of ointments, in the production of pastes as hydrogels, and in the production of drugs for parenteral use; it is of high interest as a stabilizer of silver nanoparticles. The aim of this study is to prepare stabilized silver nanoparticles in polymer films based on Na-CMC and to investigate their structures, physical and chemical properties, and bactericidal activity.
Experimental
Industrial samples of Na-CMC with DS of 0.65-0.85 and DP of 200-600 obtained from cotton cellulose were used as polymer matrices after their purification from inorganic and organic admixtures. To prepare silver nanoparticles in the films based on CMC, AgNO3 aqueous solutions of various concentrations were used. The bacterium Staphylococcus epidermidis and the yeast Candida albicans, pathogens of human and animals, were used as the test cultures. To form the films, 2-4% aqueous solutions of purified Na-CMC samples with various degrees of substitution and polymerization were employed after the removal of the gel fraction by centrifugation on a laboratory centrifuge at 2500 rpm for 20 min. Then, the calculated amounts of 0.1-0.001M aqueous solutions of AgNO3 and 0.1-0.5% glycerol, which used as a plasticizer, were added under stirring to the gel free Na-CMC solutions, and the stirring was continued until the formation of homogeneous Ag+ CMC– hydrogel. The photochemical reduction of silver ions in the Ag+ CMC– structure to nanoparticles was performed at 25 0С by their irradiation with a DRSh-250 high pressure mercury lamp. The dispersions of silver nanoparticles were prepared by ultrasonic dispersion of the hydrogels using UZDN-1 and U4.2 ultrasonic dispersers.
Results and Discussion
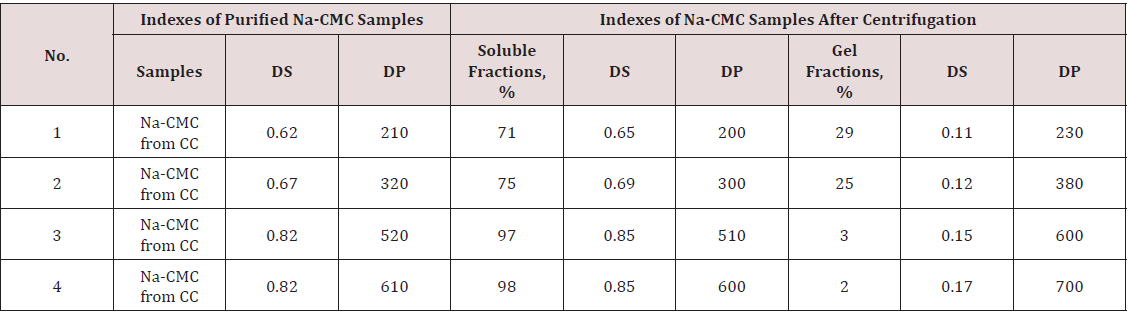
Solubility and degree of purity are the most important physicchemical characteristics of Na-CMC determine possibility of their conversion into products. We investigated composition of watersoluble and insoluble fractions of purified Na-CMC samples with various DS and DP (Table 1). As seen from the Table 1, with increasing of DS the quota of soluble Na-CMC fraction in water increased, and content of insoluble gel fraction decreased. With increase of DS the rate of gel fraction of Na-CMC in water decreased. This is probably occur due to the decreasing of the intensity of hydrogen bonds between the macromolecules of Na-CMC in increase of the DS in Na- CMC. Moreover, the compounds and properties of the gel fraction of Na-CMC depend on the type of cellulose raw material and methods for obtaining of Na-CMC. The described above investigation provided as polymeric base for the obtaining hydrogel Na-CMC containing ions and silver nanoparticles. Further studies are aimed at the formation and stabilization of silver ions and nanoparticles in Na-CMC polymer base and study their properties. At the first step of the study, Na-CMC films stabilized with silver nanoparticles were formed and photo irradiated.
Table 1: Effect of DS and DP of Na-CMC sample on quantity and composition of water-soluble and insoluble fractions.
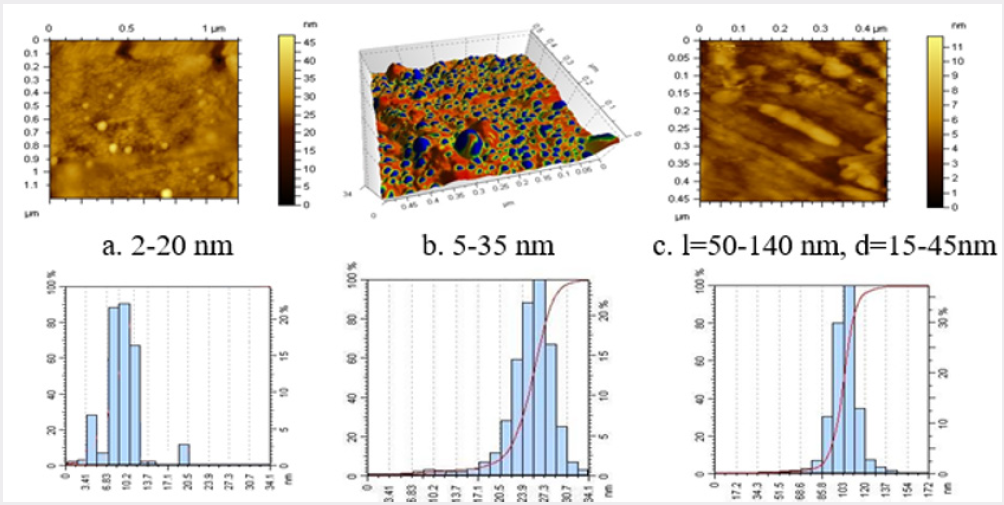
Photoreduction of silver cations at concentrations of 0.025- 2.50 wt % was performed in 2% (8∙10-2) solution of Na-CMC with a DS of 0.85 (pH 8.5), and a DP of 600. After an increase in the initial concentration of AgNO3 from 0.025 to 2.5 wt %, the UV - induced color of the Na-CMC solution was found to change from pale yellow to brown, possibly due to the increase in the amount of formed silver nanoparticles of different sizes. Meanwhile, a pure Na-CMC solution did not change color and remained clear after UV-irradiation. To confirm the formation of silver nanoparticles, electron microscopic investigations of CMC films were performed. Figure 1 shows the electron micrographs of Na-CMC films contain 0.025-2.5 wt % silver nitrate formed under UV - irradiation. To determine the forms and sizes of silver nanoparticles in Na-CMC structure the obtained samples were investigate using atomic force microscope АFМ - 5500 (Austria).
From Figure 1a, it may be concluded that, during photoirradiation at AgNO3 concentration of 0.025 wt % AgNO3, clusters and nanoparticles of silver with sizes 2-20nm are formed in the structure of Na-CMC film. After the addition of 0.25 wt.% AgNO3, 5 to 35nm spherical silver nanoparticles are formed in the structures of the Na-CMC films (Figure 1b). An increase in the silver nitrate concentration in the Na-CMC structure up to 2.5 wt % induces an increase of number of 5 to 35nm size silver nanoparticles, and rod-shaped silver nanoparticles of 50-140 nm in length and 15-45nm in width are formed simultaneously (Figure 1c). Thus, an increase in the silver ion content in the Na-CMC films leads to a relatively narrow size distribution of the spherical and rod-shaped silver nanoparticles formed during photoirradiation (Figure 1). AFM microphotograph of Na-CMC gel contained SNP (a), (b), (c), and their histograms [5].
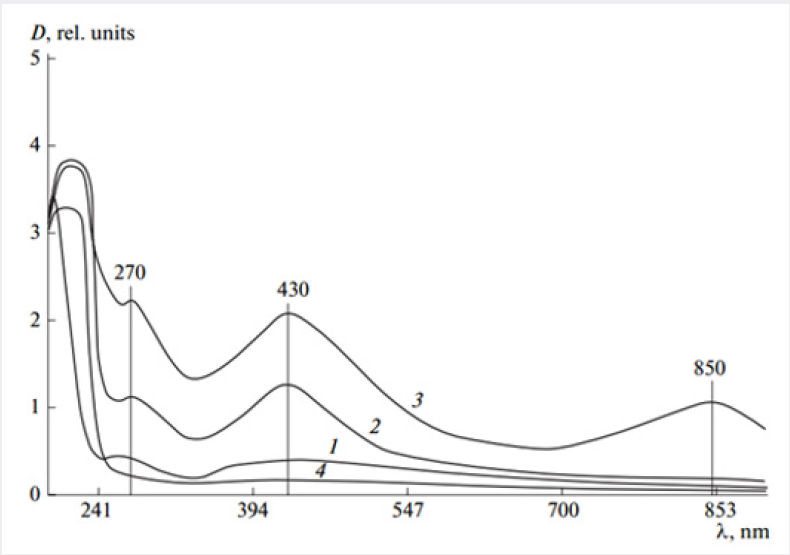
Concentration of [Na-CMC] = 8 ∙ 10-2mol; time of UV irradiation - 30min. Na-CMC films containing (a) 0.025, (b) 0.25, and (c) 2.5 wt % AgNO3. It was reported in [6] that the shapes and sizes of silver nanoparticles in various polymer films may be determined by UV spectroscopy. UV spectra of Na-CMC films prepared from the solutions with 0.025-2.5 wt % contents of silver nitrate are shown in Figure 2 shows the appearance of a maximum at λ=270nm in the spectrum at a 0.025 wt. % silver salt content in Na-CMC film (curve 1), that indicates the formation of clusters generated by dimerization of particles. In the spectrum of Na-CMC films containing 0.25 wt. % silver salt (curve 2), a new absorption band with a maximum at λ=430nm is related to the presence of 5 to 35nm silver nanoparticles [7]. At a higher concentration of silver salt (up to 2.5 wt. %) in the hydrogels subjected to photochemical reduction and subsequently film formation, the intensity of the absorption band with a maximum at λ=430 nm (curve 3) increases due to an increase of the quantity of 5 to 35 nm silver nanoparticles. In addition, a weak maximum in the near IR region of the spectrum (λ=850nm) is corresponding to exclusively rod-shaped silver nanoparticles [8] (Figure 2). The microbicidal activities of the samples of Na-CMC films containing silver cations and nanoparticles were examined in the opportunistic test cultures Staphylococcus epidermidis and Candida albicans. To identify an antimicrobial effect, the samples of the composites were placed into test tubes containing a thioglycolic medium in the case of Staphylococcus epidermidis or Saburo in the case of Candida albicans.
Figure 2: Absorption spectra of photochemically reduced samples of (1-3) Na-CMC films containing (1) 0.025, (2) 0.25, and (3) 2.5 wt % AgNO3, and (4) the film formed from the initial Na-CMC.
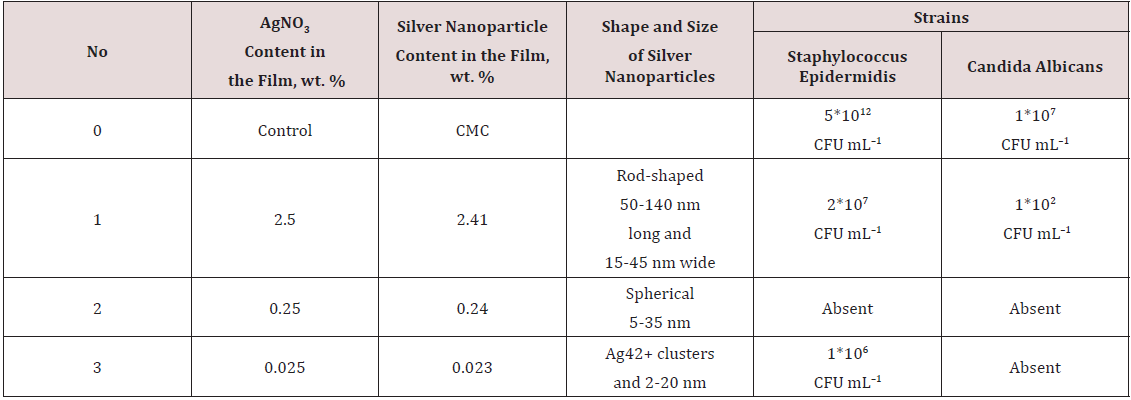
2 shows that the Na-CMC film containing 5-35nm silver nanoparticles is the most active film, it completely inhibits the growth of Staphylococcus epidermidis and Candida albicans. The samples of Na-CMC films containing relatively large (50-140nm long and 15-45nm wide) rod-shaped nanoparticles are less active than the 5 to 35nm spherical nanoparticles possibly die to their small surface areas [9] (Table 2). In addition, Na-CMC films containing Ag clusters proved to be less active then those containing 5-35nm silver nanoparticles, that may be explained by the total content of silver nanoparticles in such Na-CMC films turned out to be almost an order of magnitude less than that in the samples of films containing 5-35nm silver nanoparticles. Because the concentration of silver ions in the Na-CMC hydrogels utilized for the film formation was low (0.023%), they almost completely associated with carboxylate anions of Na-CMC. The limited mobility of ions is responsible for the lower rate of silver nanoparticle formation that seems to occur only in a “nanoreactor” structure [10].
Table 2: Comparative results of the antimicrobial activities of Na-CMC films containing silver nanoparticles of various sizes.
Conclusion
Optimal conditions of silver nanoparticles with different shapes and sizes formation in structure of CMC film with different DS and DP were determined. It was established that the replaced cations of silver in Na-CMC macromolecules subject to restoration first of all and play role of «nanoreactors» in which the negative ion carboxylic groups, according to the theory of Mott-Gurney, are the «trap» for positively charged ions of silver and processes photo stimulated formations of silver nanoparticles. UV-spectroscopic method for control of the form and sizes of silver nanoparticles at process of their restoration is developed. Formation conditions of the various shape and sizes of silver nanoparticles in dependence of parameters of components interaction reaction and photochemical restoration are revealed. Correlation dependence between the size and shape of silver nanoparticles in Na-CMC structure and their biological activity is established. It is shown that decreasing the size of the silver nanoparticles promotes the increase of their bactericidal activity at the same concentration in polymeric matrix. That indicates the difference of values of the surface of nanoparticles with the various shape and sizes. The prepared biodegradable Na-CMC films containing silver nanoparticles are of interest as bactericidal and bacteriostatic coatings for the treatment of burns and trophic ulcers.
Acknowledgement
The authors would like to thank to Laboratory of Physical – chemical Analysis for assistance in physical – chemical investigations and measurements of samples.
References
- BA Zhubanov EO, Batyrbekov RM (2000) Iskakov Polymer Materials with Therapeutic Effect Kompleks, Almaty pp. 220.
- JR Morones, JL Elechiguerra, A Camacho, K Holt, JB Kouri, et al. (2005) The bactericidal effect of silver nanoparticles. Nanotechnology V 16(10): pp. 2346-2353.
- AB Shcherbakov (2006) other Preparations of silver: yesterday today, tomorrow. Farmaseutica journal 5: 45-57.
- I Soni, B Salopek Bondi (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model of Gram-negative bacteria, J Colloid Interface Sci 27: 70-82.
- AA Sarymsakov (2005) Medium and Low Substituted Carboxymethyl Cellulose. Synthesis, Properties and Applications IKhFP Akad. Nauk Resp. Uzbekistan, Tashkent p. 179.
- AL Stepanov (2004) Optical properties of metallic nanoparticle, synthesized in polymer by method ion implantation (review) Journal technical physics 74(2): 1-12.
- LI Lopatina, VG Sergeev (2010) Influence of molecular weight and structure of polyacryl acid on formation of dark blue silver. Vestn Mosk Univ, Ser 2 Khim 51: 398-401.
- U Kreibig , M Vollmer (1995) Optical Properties of Metal Clusters.
- Kh E Yunusov, AA Atakhanov, AA Sarymsakov, S Sh Rashidova, KV Lobanova et al. (2010) Silvernanoparticles, formed on polymeric matrixes and their bactericidal properties. Pharmaceutical journal. Uzbekistan 1: 55-59.
- MV Kiryukhin, BM Sergeev, AN Prusov, VG Sergeyev (2000) Photochemical restoration cations of silver in polielectiritic matrix Polym. Sci Ser B 6(42): 1069-107

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...