Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5779
Research ArticleOpen Access 
Corrosion Protection of Copper Sculptures by Corrosive Pollutants Volume 2 - Issue 1
Rajesh Kumar Singh1*, Hema Kesavan2 and Jay Prakash Singh3
- 1Department of Chemistry, Jai Prakash University, India
- 2SRM Institute of Science and Technology, Chennai, India
- 3Department of Chemistry, Jagdam College, Jai Parakash University, India
Received: September 26, 2022 Published: October 10, 2022
Corresponding author: Rajesh Kumar Singh, Department of Chemistry, Jai Prakash University, India
Abstract
Nitrogen dioxide, carbon dioxide and sulphur dioxide are acidic gas and they produce corrosive medium for copper sculptures. These gases absorb moisture to form nitric acid, carbonic and sulphuric acid. It forms corrosion cell on the surface of copper and accelerate corrosion reactions. The corrosion cell formation is written as: Cu|Cu2+||H+|H2 thus corrosion reactions start, and copper is oxidized into Cu2+ surface and it is oxidized into Cu2+ whereas H+ ion is reduced into H2. Nitric acid environment copper exhibits galvanic, pitting, stress, crevice, blistering, embrittlement and intergranular corrosion. The corrosion reactions change physical, chemical and mechanical properties of corroded materials. Nanocoating compound tetrahydro-dibenzo[a,d] [7]annulene- 5,11-disemicarbazone and SiC electrospray compounds used to control the corrosion of copper in nitrogen dioxide medium. For corrosion mitigation of copper metal interface was coated with tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC. The coating compound tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone was synthesized in laboratory. Nozzle sprays techniques used for nanocoating and electrospraying. The corrosion rate of copper was determined by gravimetric loss method at different temperatures, concentrations, and days in nitrogen dioxide medium. Potentiation polarization technique used for the determination of electrode potential, corrosion current and current density. Nanocoating and electrospraying compounds formed a composite barrier on the surface of base metal by chemical bonding. The nanocoating and electrospray compounds adhered on base metal by chemisorptions to confirm by activation energy, heat of adsorption, free energy, enthalpy and entropy. The nanocoating and electrospray deposited on copper confirmed by Langmuir, Frundlich and Temkin isotherm. Copper formed a complex compound to interact with tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC. Electrospraying SiC blocked porosities of nanocoating compound and checked osmosis process of nitrogen dioxide. The nanocoating and electrospray compounds decreased corrosion rate and increased surface coverage areas and percentage coating efficiencies.
Keywords: Copper; Composite Barrier; Nanocoating; Electrospraying; Nozzle Spray; Thermal Parameters; SiC
Introduction
Copper metal uses for construction of sculptur es [1], monuments [2] and other items. Nitrogen dioxide develops corrosive medium copper appliances. Developed Nations expense 5% of GNP for corrosion protection materials. The acidic oxides of sulphur, carbon and nitrogen produce hostile environment of metals [3]. These gases corrosive nature [4] depend upon air quality of village [5], city and industry areas [6]. Particulate matters produce hostile environment for metals. Acids, bases and salts create ambient environment for materials [7]. The Cl- ions concentration in marine environment is high so it generates corrosive medium for building components [8]. The corrosion of metals depends upon their surrounding conditions like dry and wet weather [9], low and high temperature [10], moisture and humidity [11], nature of water [12], concentration of oxygen [13], composition of pollutants and effluents. The other factors aggravate corrosion materials like season change, weather change [14], greenhouse gases [15], global warming [16], and climate change [17] to exhibit various forms of corrosion. Solar radiation and heat produce destruction in materials [18]. Crude oil well contains CO2 and SO2 to generate hostile environment for metals. Bio-wastes develop micro and macroorganisms to cause of biological corrosion [19]. The disturbance of internal morphology of metals [20] is responsible for corrosion. Several corrosion protection techniques [21] are used for safe materials above ambient conditions. Some Protective methods [22] are applied metallic coating deposition of metal and nonmetal coating which is related to electroplating, flame spraying, cladding, hot dipping and vapour deposition process. Polymeric coating protects interface of metals [23] by paint coating and lamination of thermoplastic and thermoset plastic [24]. Organic and inorganic inhibitors suppress the corrosion of metal as per nature of surrounding environment. Organic inhibitors provide protection as anodic, cathodic and mixed inhibitors. Nanocoatings coating forms strong bonding between base meats and coating compounds but surface porosities don’t protect base metals long period. These porosities block by electrospray technique. The nanocoating and electrospray substances form composite barrier which stop osmosis or diffusion process corrosive matters. The composite barrier is passive in nature which save materials for attack of corrosive substances and enhance physical, chemical and mechanical properties of materials.
Experimental
The gravimetric, potentiostat, nanocoating and electrospray techniques used for experimental work. The nanocoating compound tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone was synthesized in laboratory.
Gravimetric technique: The test samples of copper metal cut into size of (2X4X0.1) sq cm and all samples washed distal water thus dust can be removed. The samples surface was cleaned by emery paper then after erasing with acetone. The samples were dried with hot air gun and kept into desiccators for further experiment. The copper samples dipped into 10 ml nitric acid solution and corrosion rate of uncoated and coated sample determined at 283, 293,303, 313 and 323 K temperatures and time mentioned 10, 20, 30, 40, 50 days. The samples coated with 50 mM of tetrahydro- dibenzo[a,d] [7]annulene-5,11-disemicarbazone and 20 mM of SiC. The organic compound tetrahydro-dibenzo[a,d] [7] annulene-5,11-disemicarbazone and SiC formed a thin composite barrier. Nozzle spray used coating for the covering the surface. The nanocoating and electrospray compound boned with base metal through chemical bonding to observe by values of activation energy, heat of adsorption, free energy, enthalpy and entropy.
Potentiostat: Potentiostat 173 model was used for calculation of corrosion potential, corrosion current and corrosion current density. First make an electrode of copper which kept between calomel electrode and pt electrode. The uncoated and coated sample corrosion potential, corrosion current and corrosion current density results were calculated. The sample coated with tetrahydro- dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC in 10 mM HNO3 solution.
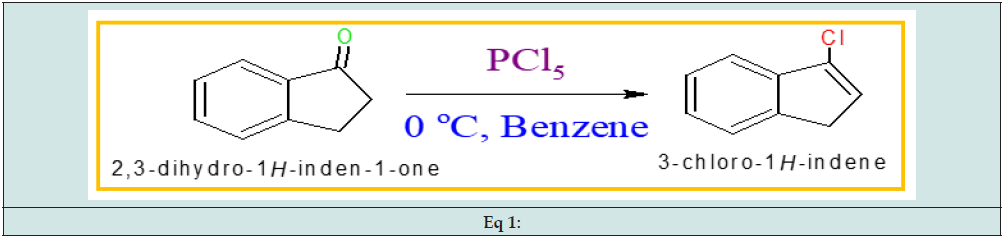
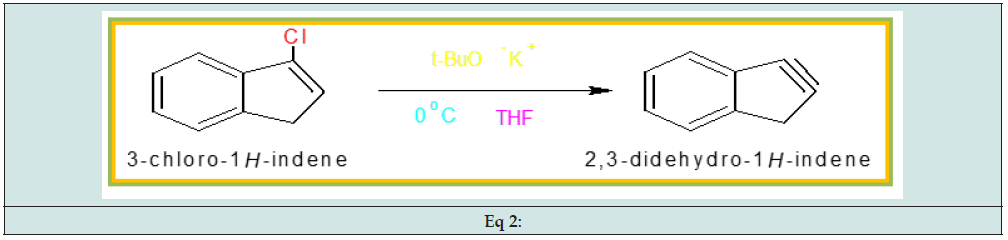
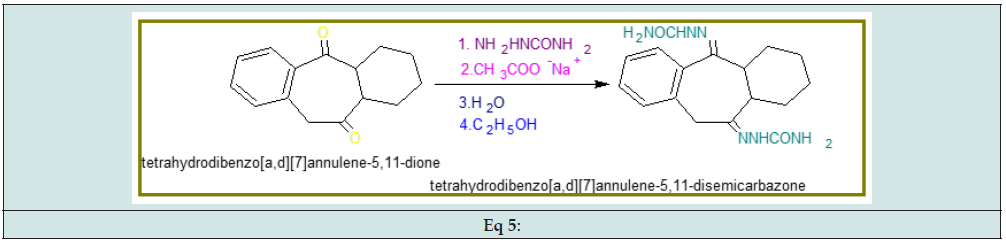
Synthesis of tetrahydro-dibenzo[a,d] [7]annulene- 5,11-disemicarbazone: Phosphorus pentachloride dissolved in benzene that solution added into 2,3-dihydro-1H-indene- 1-one at 0oC temperature to yield 3-chloro-1-H-indene. (Eq 1 Potassium t-butaoxide mixed into 3-Chloro-1H-indene solution at 0oC temperature to give 2,3-cyclohexane-indene. (Eq 2)
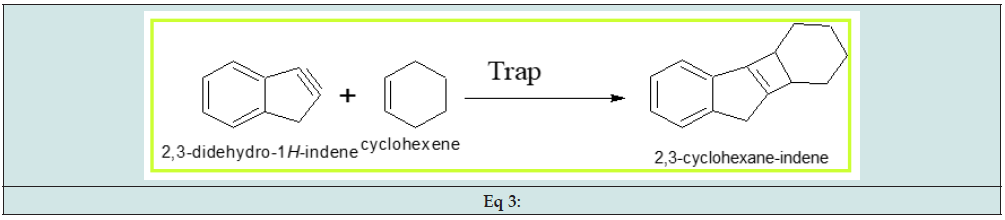
The compound 2,3-didehydro-1H-indene trapped cyclohexene to produce 2,3-cyclohexane-indene. (Eq 3)
The compound 2,3-cyclohexane-indene oxidized with NaIO4 in presence of RuO2 in solution of methyl nitrile and carbon tetrachloride to yield tetrahydro-dibenzo[a,d] [7]annulene-5,11-dione. (Eq 4)
The solution semicarbazide added into tetrahydro-dibenzo[ a,d] [7]annulene-5,11-dione in presence of sodium acetate, HCl and ethyl alcohol to reflux the reaction mixture for 1hour to yield tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone. (Eq 5).
Results and Discussion
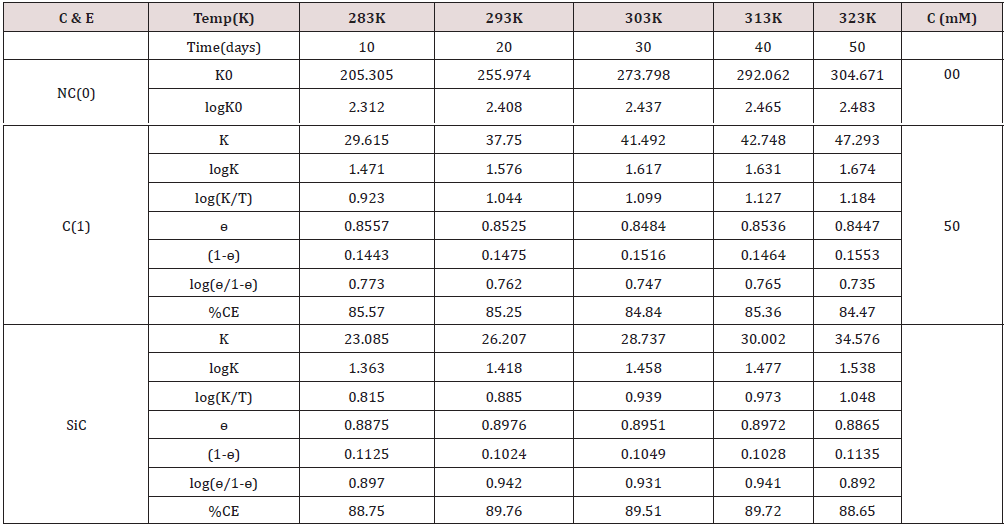
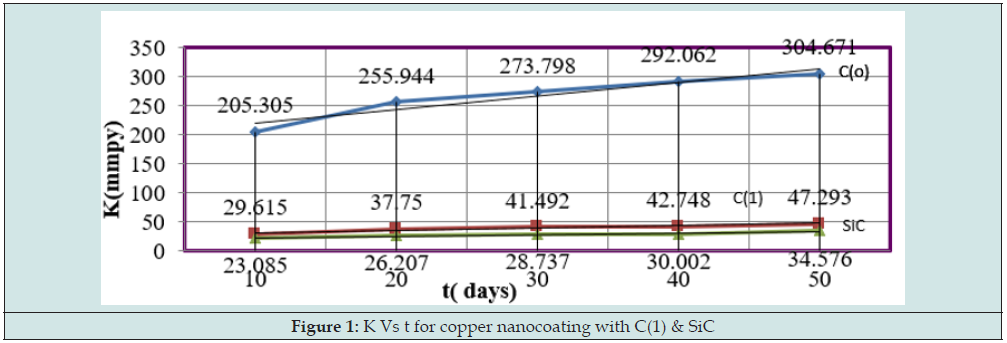
The corrosion rate of metal was calculated by equation K = 13.56 W/D A t, (where w is weight loss, D is density, A is surface area and t is immersion time). The copper metal was coated with tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC and its corrosion at different interval of 10, 20,30,40 and 50 days recorded in Table 1.
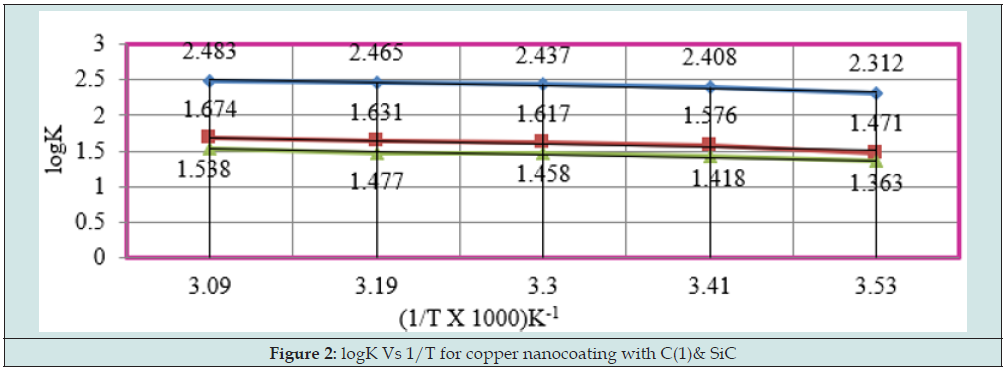
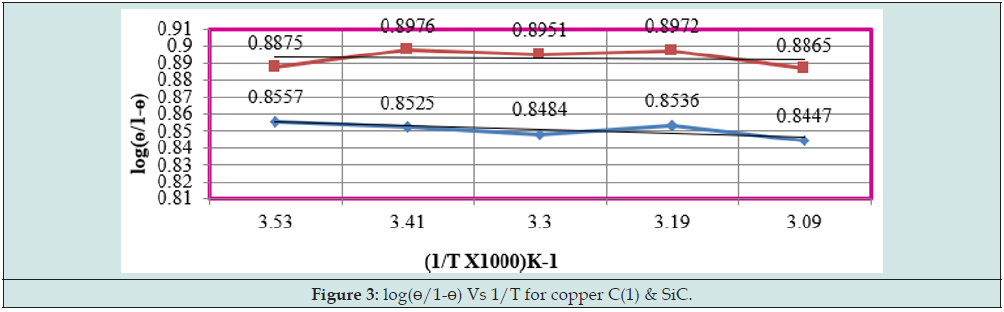
The results of Table 1 were shown that uncoated copper increased as immersion time enhanced but these reduced with coating and electrospray compounds. Such trends clearly noticed in Figure1 which plotted between K (corrosion rate) versus t (day). C(o) = Uncoated C(1) = tetrahydro-dibenzo[a,d] [7]annulene- 5,11-disemicarbazone] The corrosion rate of uncoated and coated copper was calculated at 283, 293, 303, 313 and 323K temperatures and their values were mentioned in Table 1. The uncoated copper metal corrosion rate found to be high but its values reduced coating with tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC. The corrosion rate of uncoated and coated copper variation with temperatures mentioned Table 1. It observed that tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC decreased corrosion rate as temperature rising 283 to 323 K. Figure2 plotted between logK versus 1/T found to be straight and this graph satisfied Arrhenius equation. Copper coated with tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC at 283, 293, 303, 313 and 323K temperatures and the values log(ɵ/1-ɵ) obtained by Figure 3 which plotted between log(ɵ/1-ɵ) versus 1/T. The results of log(ɵ/1-ɵ) with nanocoating and electrospray were given in Table1. The values of log(ɵ/1-ɵ) decreased with tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone but its values enhanced with SiC.
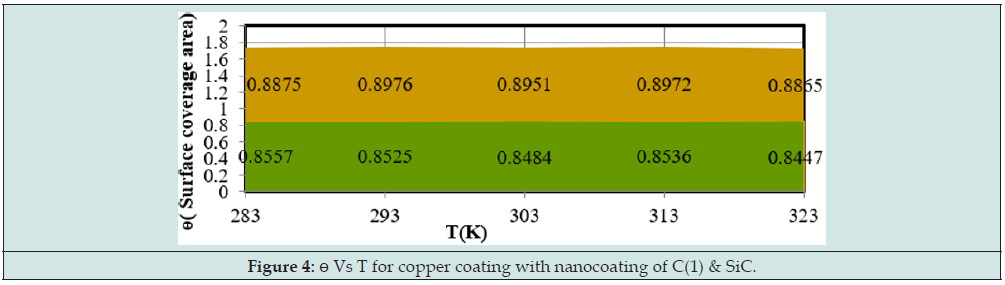
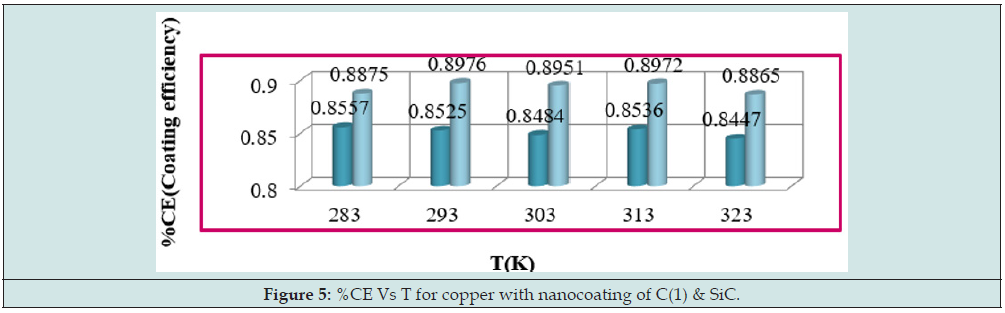
The surface coverage area developed by tetrahydro-dibenzo[ a,d] [7]annulene-5,11-disemicarbazone and SiC to calculate by equation ɵ = (Ko-Ko/K) at 283, 293, 303, 313 and 323K temperatures and their values were recorded in Table 1. It noticed that nanocoating tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone occupied lesser surface with respect of SiC . It clearly noticed in Figure 4 plotted between ɵ (surface area) versus T (temperature). The percentage coating efficiency of tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC were determined by equation CE = (Ko-Ko/K)X 100 at 283, 293, 303, 313 and 323K temperatures and their values were given in Table 1. (Figure 5) depicted SiC produced higher coating efficiencies with respect of tetrahydro- dibenzo[a,d] [7]annulene-5,11-disemicarbazone as shown in graph % CE (percentage coating efficiency) versus T (temperature).
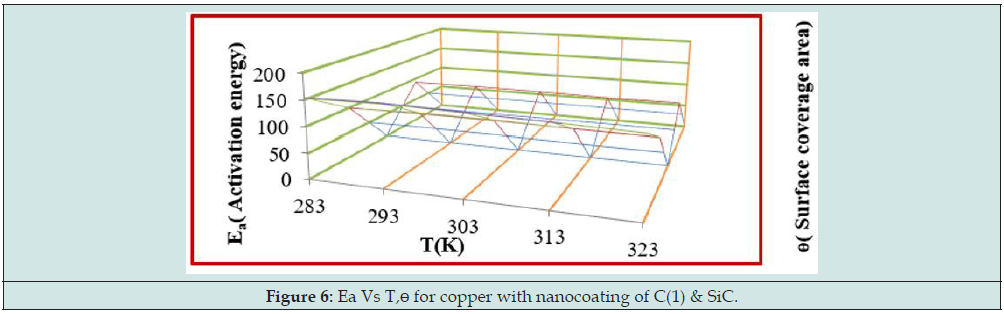
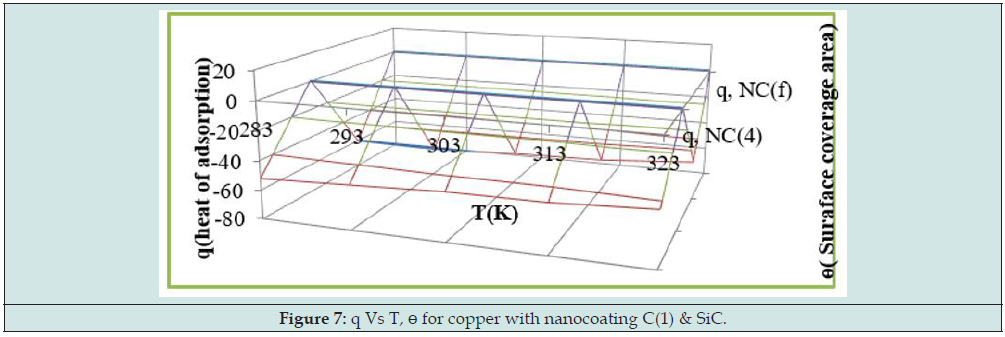
Arrhenius equation d/dt(logK) = A e-Ea/RT used and Figure 2 for calculation of activation energy of uncoated and coated copper with tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicatbazone and SiC and their values were written Table 2. Activation energy found to be higher with uncoated copper, but their values reduced with nanocoating and electrospraying compounds. Activation energies values indicated nanocoating and electrospray compounds formed chemical bond with copper metal. The activation energy results of Table 2 indicated that activation energy nanocoating and electrospray reduced, and surface coverage area increased as shown Figure 6. Heat of adsorption determined by Langmuir isotherm log (θ/ 1-θ) = log (A .C) - (q/ 2.303 R T) for copper coating of tetrahydro- dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC at 283, 293, 303, 313 and 323 K temperatures. Heat of adsorption recorded in Table 2, and its values were show that heat of adsorption reduced and surface coverage increased as shown in Figure 7 which plotted q(heat of adsorption) versus T(temperature) and ɵ(surface coverage area). The negative values of heat of adsorption indicated that nanocoating and electrospray compound bonded with copper metal by chemical bonding. Heat of adsorption determined by Langmuir isotherm log (θ/ 1-θ) = log (A .C) - (q/ 2.303 R T) for copper coating of tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC at 283, 293, 303, 313 and 323 K temperatures. Heat of adsorption recorded in Table 2 and its values were show that heat of adsorption reduced and surface coverage increased as shown in Figure 7 which plotted q(heat of adsorption) versus T(- temperature) and ɵ(surface coverage area).
Table 2: Thermal parameters values of tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicardazone and SiC.
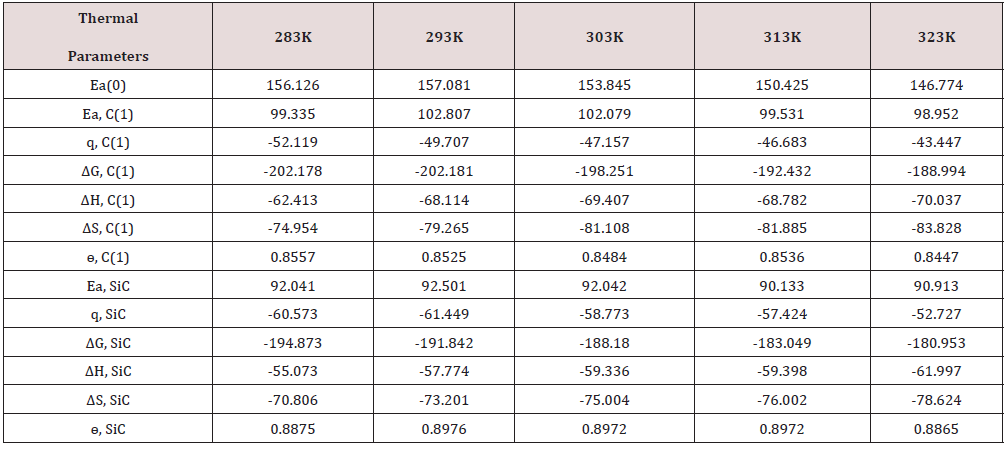
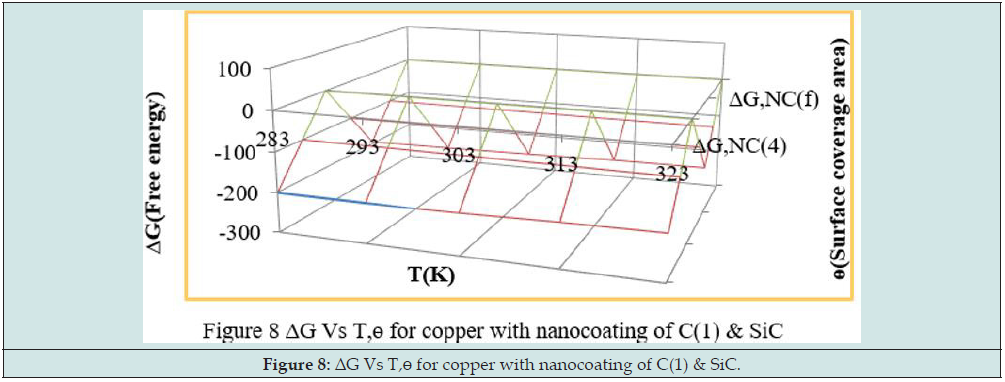
The negative values of heat of adsorption indicated that nanocoating and electrospray compound bonded with copper metal by chemical bonding. Free energy of tetrahydro-dibenzo[a,d] [7]annulene- 5,11-disemicarbazone and SiC obtained by equation ΔG = -2.303 RT log(33.3K) and their values were given in Table 2. The results of Table2 exhibited that nanocoating and electrospray formed composite barrier by chemical bonding. Figure 8 depicted that free energy decreased as temperatures rising whereas surface coverage area enhanced such results observed in Figure 8. Exothermic process occurred during nanocoating and electrospraying of tetrahydro- dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC.
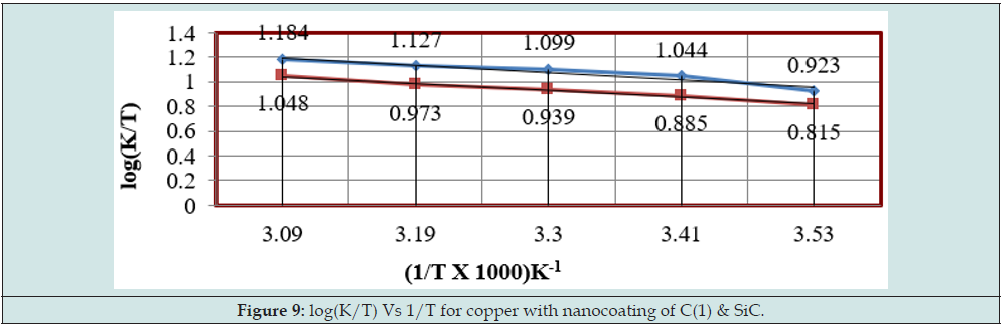
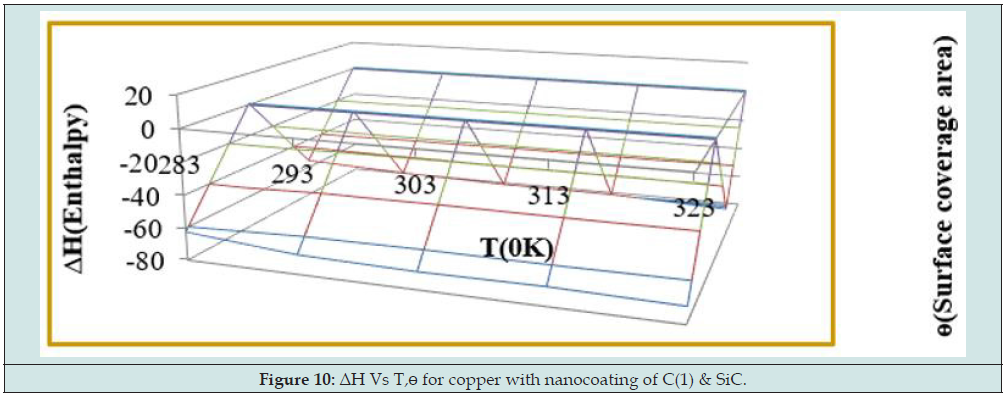
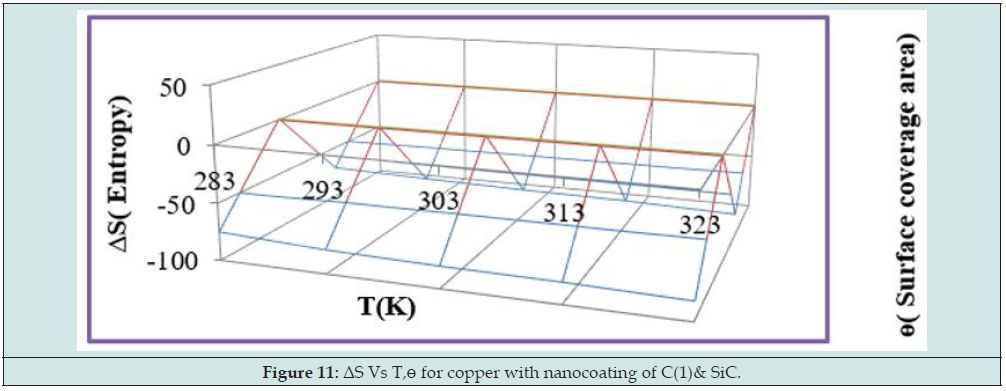
Enthalpy of tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC was calculated by transition state equation K = R T / N h log (ΔS# / R) X log (-ΔH #/ R T) and Figure 9 plotted between log(K/T) versus 1/T and their values were mentioned in Table 2. Enthalpy of nanocoating and electrospray decreased, and surface coverage area increased as shown in Figure 10. Enthalpy values found to be negative which indicated that nanocoating and electrospraying an exothermic process. Both compounds adhered on the base metal by chemical bonding. Entropy of tetrahydro-dibenzo[a,d] [7] annulene-5,11-disemicarbazone and SiC was determined at various temperatures by above mentioned transition state equation and their values were written in Table 2. The results of entropy indicated that both compounds arranged on base material in ordered manners with chemical bonding. Figure 11 plotted between ΔS (entropy) versus T, and ɵ for nancoating and electrospraying materials. Electrode potential and corrosion current density of aluminum, tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC were determined by equation ΔE/ΔI = βa βc / 2.303 I (βa + βc). The results of electrode potential and corrosion current density were written in Table 3. The results of Table 3 saw that electrode potential and corrosion current density increased with uncoated aluminum but these values were reduced by nanocoating and electrospraying materials. Anodic current enhanced and cathodic current reduced with uncoated aluminum aluminium. But it coated with tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC anodic current decreased and cathodic current increased. Corrosion current of aluminum, tetrahydro-dibenzo[a,d] [7]annulene- 5,11-disemicarbazone and SiC was determined by above equation and their values were put in equation CR (mmpy) = 0.1288 I (mA /cm2) × Eq .Wt (g) / ρ (g/cm3) to obtain corrosion rate of uncoated and coated aluminum and their values mentioned in Table 3. It noticed that corrosion rate of uncoated aluminum found to high but coated aluminum corrosion rates were low. Both materials enhanced surface coverage area and coating efficiency. The results of corrosion rate of aluminum obtained by potentiostat technique confirmed by weight loss experiment. Tetrahydro-dibenzo[a,d] [7] annulene-5,11-disemicarbazone is an electronic rich organic compound. It possesses several electron donor functional groups, so it forms thin film barrier on copper metal. This coating compound develops lots of porosities on the interface of base metal. The H3O+ ions enter inside through osmosis due to porosities form by Tetrahydro- dibenzo[a,d] [7]annulene-5,11-disemicarbazone. These ions accelerate corrosion reaction so SiC uses as electrospraying which blocked porosities and protect metal. The nanocoating compound tetrahydro-dibenzo[a,d] [7]annulene-5,11-disemicarbazone and SiC form composite thin film barrier that is work as passive barrier against corrosive medium as shown in Figures 11 & 12 .
Table 3: Potentiostat polarization of copper of tetrahydro-dibenzo [a,d] [7] annulene-5,11-disemicarbazone and electrospraying SiC.

Conclusion
The nanocoating compound Tetrahydro-dibenzo[a,d] [7]annulene- 5,11-disemicarbazone and SiC coated on the surface copper metal and its corrosion phenomena studied at different temperatures in nitrogen dioxide environment. Both compounds formed composite thin film barrier and it decreased corrosion as well enhanced surface coverage area. The results of activation energy, heat of adsorption, free energy, enthalpy and entropy depicted that composite barrier formed by chemisorptions process. These compounds adsorbed on copper were shown by plots of Langmuir, Freundlich and Temkin adsorption isotherms. These compounds increased coating efficiency and percentage coverage areas and neutralized the attack of nitrogen dioxide.
Acknowledgment
Author thanks the UGC-New Delhi for providing finical support for research work. I give my warm regards to Professor G Udaybhanu, Department of Applied Chemistry, IITD (Dhanbad), Professor IIN Nambhoothari, Department of Chemistry, IITB, Mumbai and Professor Sanjoy Misra, Department of Chemistry,R U, Ranchi, India. I am thankful my research groups to provide their support in data collection and computational results.
References
- RK Singh, Noor Alam (2019) Study the corrosion & corrosion protection of brass sculpture by atmospheric pollutants in winter season. Modern Approach on Material Science 1(3): 54-62.
- Szabo T, Molnar-Nagy L, Telegdi J (2011) Self-healing microcapsules and slow release microspheres in paints. Progress in Organic Coatings 72: 52-57.
- Videla H, LK Herrera (2009) Understanding microbial inhibition of corrosion. Electrochem Acta 39: 229-234.
- RK Singh (2017) Corrosion protection of transport vehicles by nanocoating of Dechydrobenzo[8]annulene-5,10-dihydrazone in corrosive environments and weather change. Journal of Powder Metallurgy and Mining 6(1): 1-8.
- Wen NT, Lin CS, Bai CY, Ger MD (2008) Structures and characteristics of Cr (III) based conversion coatings on electrogalvanized steels. Surf. Coat Technol 203: 317.
- Boerio FJ, Shah P (2005) Adhesion of injection molded PVC to steel substrates. J of Adhesion 81(6): 645-675.
- R K Singh, Manjay K Thakur, Sabana Latif (2018) Mild Steel corrosion control by nanocoating and filler compounds in hostile environments. J of J Material Science 4(3): 1-12.
- Deveci H, Ahmetti G, Ersoz M (2012) Modified styrenes: Corrosion physico-mechanical and thermal properties evaluation. Prog Org Coat 73: 1-7.
- Genzer J (2005) Templating Surfaces with Gradient Assemblies. J of Adhesion 81: 417-435.
- Leon-Silva U, Nicho ME (2010) Poly(3-octylthiophhene) and polystyrene blends thermally treated as coating for corrosion protection of stainless steel 304. J Solid State Electrochem 14: 1487-1497.
- Baier RE (2006) Surface behaviour of biomaterials: Surface for biocompatibility. J Mater Sci Mater Med 17(11): 1057-1062.
- RK Singh (2016) Corrosion protection of transport vehicles by nanocoating of decahydrobenzo [8]annulene -5,10-dihydrazone and SiC filler in H2O, O2 (moist): CO2, SO2 environments and weather change. Journal of Metallurgy and Materials Science 58: 167-179.
- Rao BVA, Iqbal MY, Sreehar B (2010) Electrochemical and surface analytical studies of the self-assembled monolayer of 5-methoxy-2-(octadeclthiol) benzimidazole in corrosion protection of copper. Electrochim Acta 55: 620-631.
- Liu XY, Ma HY, Hou MZ (2009) Self-assembled monolayers of stearic imidazoline on copper electrodes detected using electro chemical measurement, XPS, molecular simulation and FTIR. Chinese Sci Bull 54: 374-381.
- Liao QQ, Yue ZW, Zhou Q (2009) Corrosion inhibition effect of self-assembled monolayers of ammonium pyrrolidine dithiocarbamate on copper. Acta Phys Chin Sin 25: 1655-1661.
- Zhang DQ, He XM, Kim GS (2009) Arginine self-assembled monolayers against copper corrosion and synergitic effect of iodide ion. J Appl Electrochem 39: 1193-1198.
- Ghareba GS, Omanovic S (2010) Interaction of 12-aminododecanoic acid with a carbon steel surface: Towards the development of ‘green’ corrosion inhibitors. Corrosion Sci 52: 2104-2113.
- Sahoo RR, Biswas SK (2009) Frictional response of fatty acids on steel. J Colloid Interf Sci 333: 707-718.
- Raman R, Gawalt ES (2007) Selfassembled monolayers of alkanoic acid on the native oxide surface of SS316L by solution deposition. Langmuir 23(5): 2284-2288.
- RK Singh (2015) Building materials corrosion control by fiber reinforced polymers, Journal of Powder Metallurgy and Mining 4(2): 1-5.
- Li DG, Chen SH, Zhao SY (2006) The corrosion Inhibition of the self-assembled Au and Ag nanoparticles films on the surface of copper. Colloid Surface A 273(1-3): 16-23.
- Cristiani P, Perboni G, Debenedetti A (2008) Effect of chlorination on the corrosion of Cu|Ni 70|30 condenser tubing. Electrochim Acta 54(1): 100-107.
- Cristiani P (2005) Solutions fouling in power station condensers. Appl Therm Eng 25: 2630-2640.
- RK Singh, Rajeev Kumar (2014) Study corrosion and corrosion protection of stainless steel in phosphate fertilizer industry. American Journal of Mining and Metallurgy 2: 27-31.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...