
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Research Article(ISSN: 2641-1725) 
Virtual Screening Of N’-Methylene-1H-Indole- 2-Carbohydrazide Schiff ‘s Base Derivatives as Cyclooxygenase Inhibitors Volume 4 - Issue 2
Amira A Sadawe1, Omran Fhid1, Inass A Sadawe1, Nisreen H Meiqal1, Abdulathim A A Alshoushan2, Salah M Bensaber1, Anton Hermann3 and Abdul M Gbaj1*
- 1Department of Medicinal Chemistry, Faculty of Pharmacy, University of Tripoli, Libya
- 2National Centre for Food and Drug Control (LFDA), Tripoli, Libya
- 3Department of Biosciences, University of Salzburg, Salzburg, Austria
Received: December 20, 2019; Published: January 07,2020
*Corresponding author: Abdul M Gbaj, Associate Professor of Genetics and Biochemistry, Department of Medicinal Chemistry, University of Tripoli, Libya
DOI: 10.32474/LOJMS.2020.04.000185
Abstract
A number of Schiff’s base derivatives for N’-methylene-1H-indole-2-carbohydrazide have been designed and evaluated in-silico for their cyclooxygenase (COX) inhibition ability. All molecules were docked to the active site of both COX-1 and COX-2 using the Auto Dock 4.2 software. All studied compounds showed high docking score against both COX-1 and COX-2. The docking scores and binding energy of the investigated compounds were found to be very promising as cyclooxygenase inhibitor and consequently as non-steroidal analgesic agents.
Keywords: Non-steroidal anti-inflammatory; molecular docking; Schiff`s base; cyclooxygenase
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a miscellaneous class of drugs usually used for the treatment of inflammatory conditions, analgesia and fever with little side effects to gastrointestinal tract as well as alleviating the pains of daily life. Consequently, there is an increasing attention in the research and expansion of selective COX-2 inhibitors [1-4]. Cyclooxygenase inhibition could be achieved by NSAIDs, where they interrupt the formation of prostacyclin, thromboxane and prostaglandins from arachidonic acid, this interruption results in mild analgesia, antipyretic action, some anti-inflammatory effects and antiplatelet effects. It has been shown that there are distinctive forms of cyclooxygenase [5,6], the constitutively expressed form (usual for homeostasis) is referred to as COX-1, and the inducible form (in response to injury) is referred to as COX-2. COX-1 is found in platelets, GI mucosal cells, and renal tubule cells, while COX-2 has been identified in chondrocytes, fibroblast, macrophages, endothelial cells, and mesangial cells. COX-2 is induced by exposure to a variety of mitogens cytokines, and endotoxin, and it is upregulated at inflammation sites [7-10].
Many indole derivatives were synthesized and evaluated for their analgesic activity, anti-inflammatory activity through cyclooxygenase inhibition activities [11,12]. The indole derivatives have shown significant anti-inflammatory activity as compared to the reference drug indomethacin. In addition, some indole derivatives were found to be selectively inhibiting COX- 2 expression and providing the gastric sparing activity [13,14]. Molecular docking is an smart scaffold to realize drug-biomolecular interactions for the rational drug design and discovery, as well as in the mechanistic study by placing a molecule (ligand) into the favorite binding site of the target precise region of the protein / DNA (receptor) principally in a non-covalent style to form a stable complex of probable efficacy and more specificity [15-17]. The information gained from the docking performance can be used to propose the free energy, binding energy and stability of complexes. At present, docking technique is used to forecast the provisional binding parameters of ligand-receptor complex in advance. The core objective of molecular docking is to achieve ligand-receptor complex with optimized conformation and with the meaning of possessing less binding free energy. The total predicted binding free energy is revealed in terms of various parameters, electrostatic (ΔGelec), torsional free energy (ΔGtor), hydrogen bond (ΔGhbond), desolvation (ΔGdesolv), dispersion and repulsion (ΔGvdw), total internal energy (ΔGtotal) and unbound system’s energy (ΔGunb). Consequently, the predicted binding free energy (ΔGbind) offers extra evidences about the nature of a variety of kinds of interactions leading to the molecular docking [18-20]. The aim of this work is to develop new inhibitors of cyclooxygenase (COX1 and COX2) using molecular modelling studies.
Materials and Methods
Molecular docking
The starting geometry of the N’-methylene-1H-indole-2- carbohydrazide Schiff’s base derivatives was constructed using chem3D Ultra (version 8.0, Cambridge soft Com., USA). The optimized geometry of N’-methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives with the lowest energy was used for molecular docking. Crystal structures of COX1 in a complex with a transition-state analogue (2OYE) was downloaded from Protein Data Bank (https://www.rcsb.org/structure/2oye) and for COX2 was downloaded from Protein Data Bank (https://www.rcsb.org/ structure/4COX). Molecular dockings of N’-methylene-1H-indole- 2-carbohydrazide Schiff’s base derivatives with COX1 and COX2 was accomplished by Auto Dock 4.2 software from the Scripps Research Institute (TSRI) (http://autodock.scripps.edu/). Firstly, polar hydrogen atoms were added into protein molecules, then partial atomic charges of the COX1 and COX2 enzymes and N’- methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives molecules were calculated using Kollman methods [21]. In the process of molecular docking, the grid maps of dimensions (62Å X 62Å X 62Å) with a grid-point spacing of 0.376Å and the grid boxes is centered. The number of genetic algorithm runs and the number of evaluations were set to 100, all other parameters were default settings. Cluster analysis was performed on the docking results by using a root mean square (RMS) tolerance of 2.0Å, which was dependent on the binding free energy. Lastly, the dominating configuration of the binding complex of N’-methylene- 1H-indole-2-carbohydrazide Schiff’s base derivatives with COX1 and COX2 enzymes fragments with minimum energy of binding were determined which relied strongly on the information of 3D structures of the COX1 and COX2 binding site and ultimately generated a series of COX1 and COX2 binding complex.
Results and discussion
Molecular docking analysis
Table 1 shows the binding energies of N’-methylene-1H-indole- 2-carbohydrazide Schiff’s base derivatives and COX1 (2OYE) and COX2 (4COX) obtained by the molecular docking strategy. In this study, molecular dockings of the N’-methylene-1H-indole-2- carbohydrazide Schiff’s base with COX1 (2OYE) and COX2 (4COX) were performed using Auto Dock 4.2 to investigate the binding mode of N’-methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives with COX1 (2OYE) and COX2 (4COX) and to obtain information about interaction forces between N’-methylene- 1H-indole-2-carbohydrazide Schiff’s base derivatives and COX1 (2OYE) and COX2 (4COX) enzymes. N’-methylene-1H-indole-2- carbohydrazide Schiff’s base derivatives and COX1 (2OYE) and COX2 (4COX) were kept as flexible molecules and were docked into seven forms of rigid COX1 and COX2 to obtain the preferential binding site to N’-methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives on both enzymes.
The docking results showed that there are many types of intermolecular interactions were involved in the binding between N’-methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives with COX enzymes, these includes Van Der Waals, hydrogen bonding and electrostatic interactions. The contribution of Van Der Waals and hydrogen bonding interaction is much greater than that of the electrostatic interaction because the sum of Van Der Waals energy, hydrogen bonding energy and desolvation free energy is larger than the electrostatic energy, which is consistent with the literature [22,23]. N’-methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives and celecoxib and COX2 (4COX) interactions are shown in Figure 1. N’-methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives showed a good binding energy as shown in Table 1. The N’-methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives showed good binding affinities for both COX1 and COX2 when compared to standards indomethacin (-6.83 and -10.16, kcal/mol), Celecoxib for COX2 (-8.76 kcal/mol) and IM8 for COX1 (-3.42 kcal/mol). Figure 1 showed eight hydrogen bonds between celecoxib and COX2 while Cp1 six hydrogen bonds with COX2. In addition, Cp1 showed good docking interaction with the COX2 binding site (ARG 120, TYR387 and ALA527) (Figure 1) and similarly celecoxib showed good docking interaction with the COX2 binding site (MET522, TRP387, ALA527, SER530 and LEU531). The interaction of N’-methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives with the COX1 and COX2 binding site of the enzyme is essential for effective inhibition as previously reported for Gel [24-26]. Therefore, N’-methylene-1H-indole-2- carbohydrazide Schiff’s base derivatives may be considered as the effective nonselective cyclooxgenase enzyme inhibitors. Important molecular properties of the investigated N’-methylene-1H-indole- 2-carbohydrazide Schiff’s base derivatives e.g., molecular weight, the number of hydrogen bond donors, the number of hydrogen bond acceptors, and log P, have been calculated. These parameters can be used to estimate whether N’-methylene-1H-indole-2- carbohydrazide Schiff’s base derivatives have properties that would make it a likely orally active drug, according to the Lipinski’s rule of five [27,28]. The number of violations of the Lipinski rules allows evaluating drug likeness for N’-methylene-1H-indole-2- carbohydrazide Schiff’s base derivatives Table 1.
Table 1: Various energies in the binding process of N’-methylene-1H-indole-2-carbohydrazide Schiff’s base derivatives and COX1 (2OYE) and COX2 (4COX) obtained from molecular docking. The unit of all energies (ΔG) is kcal/mol.
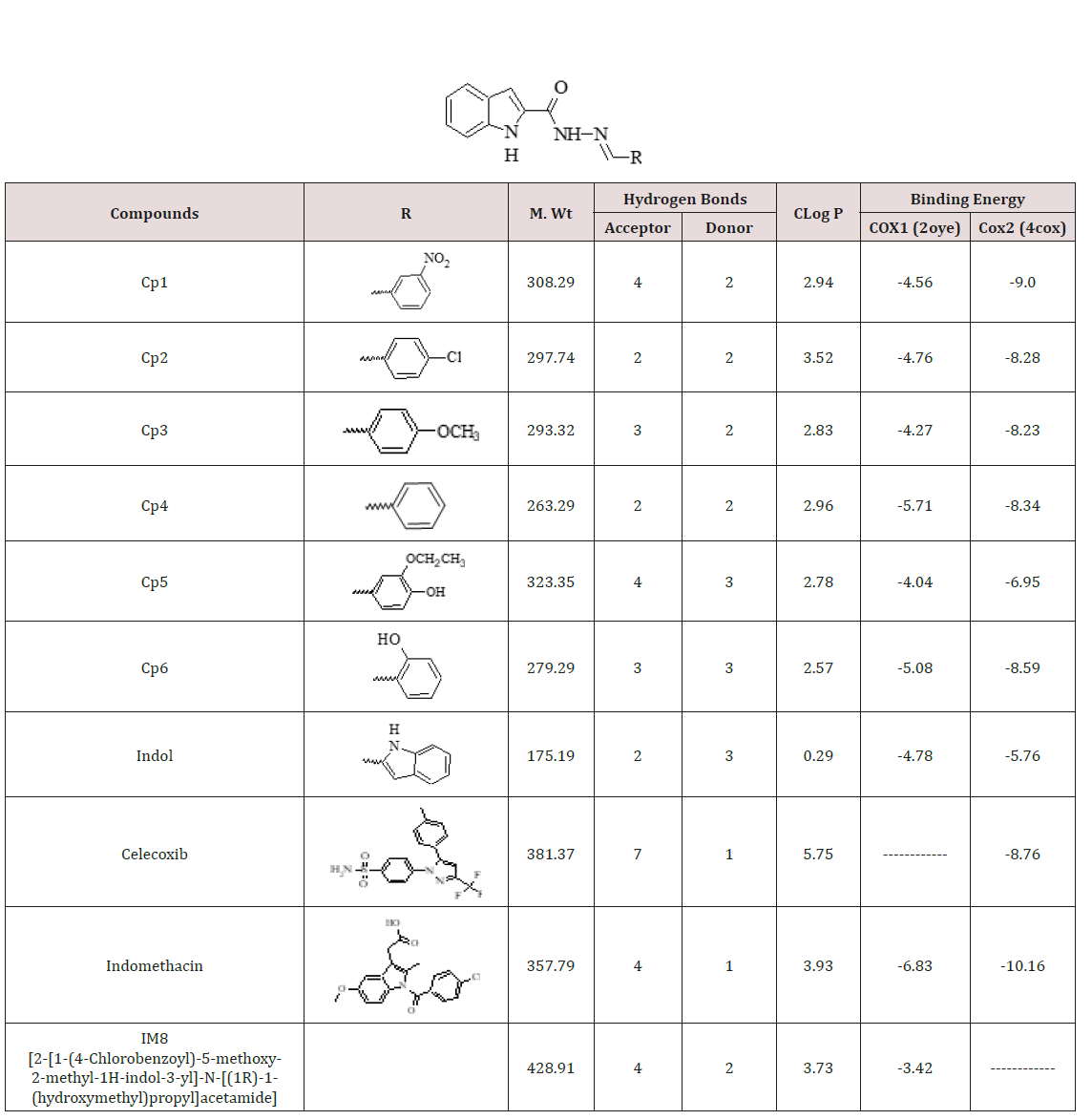
Figure 1: Shows the interaction model between celecoxib (A) and Cp1 (B) with COX2 (4COX) active site. Celecoxib (A) and Cp1 (B) molecules were shown in green colour. The hydrogen bonds are represented using green broken line.
References
- Garet Martin, Boudet Gil, Montaurier Christophe, Vermorel Michel, Coudert Jean, et al. (2004) Estimating relative physical workload using heart rate monitoring: a validation by whole-body indirect calorimetry. 94(1-2): 46-53.
- John Edison Muñoz (2016) Workload Management Through Glanceable Feedback: The Role of Heart Rate Variability.
- Arana Munarriz Victor, Echeberria Aizpuru Iñaki (1999) Monotorización de la frecuencia cardíaca en el estudio de la carga física en el trabajo.
- Keyzerling MW (1988) “Postural Analysis in Industry”. Ergonomics in Manufacturing Raising Productivity through Workplace Improvement. Society of Manufacturing Engineering. The United States of America.
- Balderas-López M, Zamora-Macorra M, Martínez-Alcántara S (2019) Musculoskeletic disorders in workers of tire manufacturing: analysis of the work process and risk of the activity. Acta Universitaria 29, e1913.
- Bingham SA, Goldberg GR, Coward WA, Prentice AM, Cummings JH (1988) The effect of exercise and improved physical fitness on basal metabolic rate. Br J Nutr 61(2):155-173.
- Brighenti-Zogg Stefanie, Mundwiler Jonas, Schupbach Ulla, Dieterle Thomas, Wolfer David Paul, et al. (2016) Physical Workload and work capacity across occupational groups. PLoS One 11(5): e0154073.
- De la Iglesia HA, Gomez BJ, Saenz AR, Ruiz FC, Carmona BA, et al. (1994) Carga de trabajo físico y costo cardiaco: La frecuencia cardiaca de referencia.
- Korshøj Mette, Krustrup Peter, Jørgensen Marie Birk, Prescott Eva, Hansen Åse Marie, et al. (2012) Cardiorespiratory fitness, cardiovascular workload and risk factors among cleaners; a cluster randomized worksite intervention. BMC Public Health 12: 645.
- Oude Hengel Karen M, Blatter Birgitte M, Joling Catelijne I, Van der Beek Allard J, Bongers Paulien M (2012) Effectiveness of an intervention at construction worksites on work engagement, social support, physical workload, and need for recovery: results from a cluster randomized controlled trial. BMC Public Health 12:1008
- Pannnemans Daphnel LE, Westerterp Klaas R (1994) Energy expenditure, physical activity and basal metabolic rate of elderly subjects. Br J Nutr 73(4): 571-581.
- Speakman John R, Selman Colin (2003) Physicla activity and resting metabolic rate. Proc Nutr Soc 62(3): 621-634.
- Rattanamanee Tarit, Nanthavanij Suebsak, Dumrongsiri Aussadavut (2014) Multi-workday vehicle routing problem with ergonomic consideration of physical workload. The International Journal of Advanced Manufacturing Technology 76(9-12): 2015-2026.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




