Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Review Article(ISSN: 2641-1725) 
Spectrum of Thyroid Cancers & its Variants: An Exhaustive Analysis of the Literature Volume 6 - Issue 2
Fatimah Kharal1, Sakshi Bai2, Muhammad Hassan Hafeez3, Muhammad Shikaib4, Ahmed Abdullah4, Sharmeen Naz5, Sarah Khalid6, Muhammad Ali Khan7* and Fahmeeda Khatoon Junejo7
- 1Department of Medicine, Combined Military Hospital, Lahore, Pakistan
- 2Department of Medicine, Jinnah Sindh Medical University, Karachi, Pakistan.
- 3Department of Medicine, Shalamar Hospital, Lahore, Pakistan
- 4Department of Medicine, King Edward Medical College Lahore, Pakistan
- 5Department of Family Medicine, Dow University of Health Sciences, Karachi, Pakistan
- 6Department of Dermatology, Baqai Medical University, Karachi, Pakistan
- 7Department of Pathology, Jinnah Sindh Medical University, Karachi, Pakistan
Received: September 12, 2022 Published: September 16, 2022
*Corresponding author: Muhammad Ali Khan, Department of Pathology, Jinnah Sindh Medical University, Karachi, Pakistan
DOI: 10.32474/LOJMS.2022.06.000235
Abstract
The Thyroid Carcinomas (TCs) have 5 main histological types: papillary, follicular or differentiated, poorly differentiated, anaplastic (the most destructive type), and medullary TC. Significant variability has been observed, both among and within neoplasms, in these different types of TCs. The first 4 kinds of TCs arise from thyroid follicular cells exhibiting significant variability. It is noteworthy that this variability encompasses histo-pathological variation, difference in the quantities of interactions between the tumor and neighboring microenvironment and inter-patient dissimilarities. Such elements give rise to pronounced complexity in tumor development from malignant cells. The present review summarizes the knowledge regarding heterogeneity of TCs. Furthermore, such research can help to attain improved conception of the basic structures giving rise in growth and TCs variability, hence contributing towards more effective management approaches.
Keywords: Carcinoma; Thyroid Carcinoma; Papillary Thyroid Cancer; Follicular Thyroid Cancer; Differentiated Thyroid Cancer; Anaplastic Thyroid Cancer; Medullary Thyroid Cancer
Introduction
The most prevalent endocrine cancer, Thyroid Carcinoma (TC), affects the parenchymal cells of the thyroid and accounts for 3.5% of all cancer cases identified each year [1]. The thyroid parenchyma is made up of of two main cell kinds: (a) the thyroid follicular cells accounting for Differentiated Thyroid Cancer (DTC) and (b) the parafollicular cells (also called C-cells) accounting for Medullary Thyroid Carcinoma (MTC). DTC includes Papillary Thyroid Cancer (PTC), Follicular Thyroid Cancer (FTC) and Hurthle cell cancer accounting for approximately 95% of all thyroid cancers; MTC comprises of approximately 1 - 2%, and anaplastic thyroid cancer comprises of approximately < 1% of all thyroid malignancies [2,3].
The World Health Organization (WHO) classification 2017 restructured the diagnostic standards and genetic and molecular features of the thyroid tumors for biology and activities of such tumors; in this, the type of borderline cancer or unclear malignant potential were also introduced [4]. As per WHO classification, 5 major histological TC types are defined: PTC, FTC, poorly differentiated TC (PDTC), MTC and Anaplastic Thyroid Carcinoma (ATC) [5]. Among these, Differentiated TCs (DTCs) include PTC and FTC accounting for 80 and 10% of all TC cases respectively. The DTCs can give rise to further poorly differentiated and toxic undifferentiated types of TC. MTC develops in the stromal microenvironment arising from C cells and exhibits clinical progress and molecular grounds that are entirely different from those of non-MTCs [6].
The utmost acknowledged theory of follicular carcinogenesis is alteration of thyroid follicular cells into differentiated or undifferentiated malignancy [7,8]. Here, diverse molecular changes are linked with precise phases, resulting in development of undifferentiated follicular-derived thyroid carcinomas from well-differentiated TCs. Also, the malignant stem-like cells theory was suggested, conferring to generation of phenotypically diverse malignant cells by a minor subpopulation of stem cells, ensuing genetic and epigenetic modifications [9,10]. PDTC and ATC are infrequent tumors accounting for 5% and 1% cancers, respectively; these exhibit destructive behavior and petite median survival time i.e. 5 years and 6 months, respectively [8].
Methodology
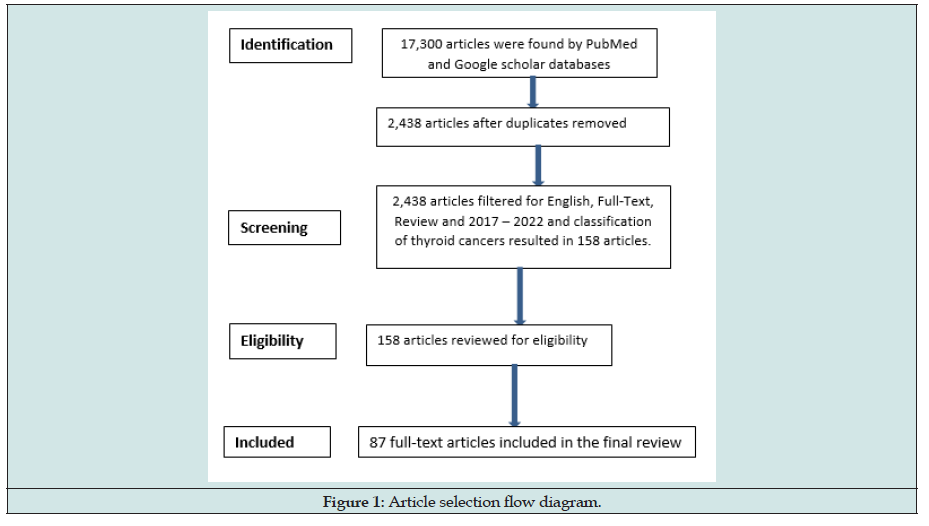
The literature analysis was done via PubMed and Google Scholar for published studies and articles from 2017 - 2022, using the keywords: thyroid cancers, classification of thyroid cancers and heterogeneity of thyroid cancers, which resulted in 17,300 articles. Only those abstracts were reviewed that related to general overview on thyroid cancers. Articles included were published in English, had full text availability and provided sufficient information on general overview of TC and its subtypes (i.e. 87 articles) (Figure 1).
Discussion
Etiology
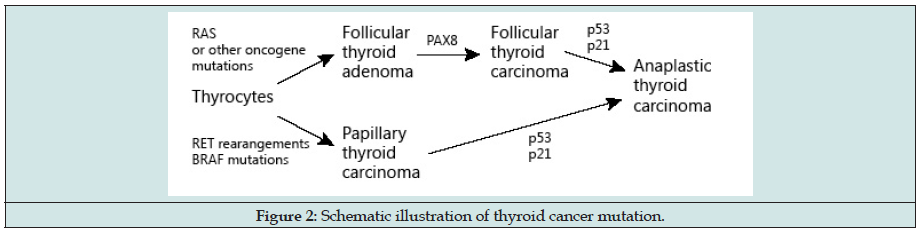
The hereditary manifestation of thyroid carcinoma is about 15 to 30% for MTC and 5% for PTC and FTC [11]. In the genetic basis of most thyroid malignancies, variations and relocations in the genes coding have been involved [12-15]. Numerous other uncommon gene mutations e.g. TERT mutations, have been related to the progression of thyroid cancer, particularly highly destructive PTC [16- 21]. DTC may be inherited by autosomal dominance or may arise as a part of tumor-susceptibility syndromes [22] (Figure 2).
Risk Factors
The major risk factors for DTC are female gender [23], exposure to radiation of the thyroid gland and a history of thyroid cancer in bloodline [3,24].
Mechanism of Thyroid Cancer
Due to the genome sequence availability, much advancement has been observed in explicating the molecular mechanisms for TC in the past 3 decades [8,25]. Genetically TC is a simple illness having somewhat little burden of somatic mutation in every neoplasm. Driver mutations which deliver a selective growth benefit thereby stimulating cancer progression; have been observed in>90% TCs [8]. The progression of TC and dedifferentiation to PDTC and ATC implicates several other mutations influencing different cell signaling pathways like p53 and Wnt/β-catenin. Moreover, TERT promoter mutations are defined in all the histological types of TC, with a considerably greater prevalence in destructive and undifferentiated neoplasms, signifying their part in TC development [26]. For most MTC cases, there are RET (Rearranged during transfection) proto-oncogene mutations. In the multiple endocrine neoplasia type 2A (MEN2A) and 2B (MEN2B) disorders, the mutations can also occasionally occur or as a result of genetic germ line processes. H-, K-, and N-RAS mutations are responsible for very few sporadic MTC [8].
Heterogeneity of Thyroid Cancers
There is extensive heterogeneity of TC cases amid all cancers: ranging from a thyroid papillary micro carcinoma (incidence reported up to 30% in healthy individuals) to ATC, one of the most fatal malignancies that become fatal in massive cases even though resected radically at preliminary phase. Certain subtypes of TCs were also categorized from benign to cancerous e.g. Non-Invasive Follicular Thyroid Neoplasm with papillary-like nuclear characteristics (NIFTP). Several research have been conducted to explicate the heterogeneity of various common malignancies like melanoma and cancers of breast, lung, colon, etc., yet TCs have been seldom addressed [6].
Papillary Thyroid Carcinoma
PTC neoplasms mostly have an infiltrating parenchyma and asymmetrical borders. Variation within a histological class exhibits multiple foci in the thyroid. When this diversity is identified, it may not demonstrate the level of molecular heterogeneity [27,28]. PTC seldom exhibits as a homogenous neoplasm microscopically. The typical variant is frequently complemented by the follicular variant. This co-occurrence of the histological variants may be clinically pertinent if one of them signifies a more destructive form i.e. the Tall-cell Variant (TCV). The TCV is involved as the dominant variant in 10% PTCs only with signs of an aggressive variant. Nevertheless, relapses and metastases in these circumstances can be linked with a greater percentage of TCV. With reports of 10-70% TCV cells, regarding the smallest region of tall-cell morphology required to diagnose TCV, the literature is divided. At present, TCV in PTC is taken into consideration when at the minimum 50% of the cells display typical TCV morphology [6,29-31].
Another aggressive variant of PTC is HV found in heterogeneous PTC cases [32]. A case series revealed that the HV-PTC type classically denotes more than 30% of the tumor [30]. Distant bone, liver, brain and lung metastases have been found in more than 50% of all established cases of HV-PTC. In research performed by Chung et al., the classical blunt projected (hobnail) characteristics of PTC arose as signs that were intensely related to LNM risk [33]. If tumors are exposed apically localized cuboidal-to-oval cells, producing a luminal development in more than 5% of a given tumor section, then they are categorized as PTCs with hobnail features. Such heterogeneous tumors are often existent with LNMs. A higher risk of LNMs is indicated when there is nonexistence of fibro vascular arrangements in > 20% of the neoplasm section but presence of micro papillary structures < 200 μm in size [33].
Mostly, PTCs are described through the existence of more than 1 anatomically alienated focus; this is defined in 18-87% of established PTC cases [6]. Still, there is a deficiency of agreement if this existence is due to several independent tumors or owing to the intra thyroidal spread from a primary sole tumor yet, the two hypotheses were established in several studies, as reported by Kuhn, et al. The general conclusion, despite of the variances in molecular techniques between these research, was that PTC diversity could be due to either intra thyroidal spread or multicentricity [34]. Above all, an interesting result was display of the similar X chromosome inactivation form by ipsilateral PTC foci with inconsistency noticed amid contralateral nodules [34].
Follicular Thyroid Carcinoma
After PTC, FTC is the frequently seen TC. FTCs are more aggressive than PTCs; they are at a more advanced phase at diagnostic time, are not as much sensitive to customary treatment and lead deaths frequently. The pre-operative FTC identification is very challenging due to resemblance to FAs. FTC and FA are the same histopathologically and demonstrate a common genetic context [35,36]. In FTCs and FAs, RAS somatic mutations and PAX8/ PPARγ reordering, and the main FTC modifications were observed [37]. As compared to PTCs, FTCs display comparatively less phenotypic heterogeneity; about 80% of FTC cases encompass micro follicular or solid/trabecular development and about 20% of cases show well-developed colloid-containing follicles. Metastases were displayed by just a minor group of patients presenting monotonous follicular tumors with capsular incursion; this could be elucidated by heterogeneity at molecular stage [6].
Regardless of its more destructive nature, FTCs heterogeneity has not been researched broadly as like PTCs. Apart from the stated genetic modifications, numerous other genes have revealed mutations in FTC, though with a much inferior occurrence, comprising DICER1, PTEN, EZH1, SPOP and PI3CA [38,39]. Several of these genes have role in the PTEN/ AKT/PI3K pathway, which is considered as main signaling pathway in FTC creation [40]. They described 2 chromosomal gains existing in all compartments (11q and 17q), proposing that these might be initial events in tumor growth, and revealed that chromosomal gains were extra common than the losses [41].
Poorly Differentiated and Anaplastic Cancers
An incomplete tumor capsule with extensive progression is characteristic of poorly differentiated carcinomas [42]. Monomorphic cancer cells can display a heterogeneous formation accompanied by trabecular, solid and insular forms in most of the cases, microscopically. In uncommon cases, the key tumor may be seen with nodular satellites. When a well-differentiated cancer dedifferentiates, it may look like a colloidal tumor that is well-differentiated macroscopically and is nearly bordered by a solid tumor with necrosis which is the poorly differentiated element [6]. There is low chance of dedifferentiation of a well-differentiated cancer [43].
PDTC is described by intensely decreased lymphocyte and dendritic cell infiltrates and an enhanced quantity of Tumor-related Macrophages (TAMs). The most common genetic variations in PDTC are TP53 gene mutations, reflecting chromosome instability and TC advancement [6,44]. As RET/PTC and PAX8/PPRγ reordering and features of DTCs are seldom seen in PDTC so it is suggested that such mutations are not linked with cell dedifferentiation [45]. RAS changes with metastatic capability may exist that have also been defined in PDTC. BRAF mutations are fairly common in PDTC related to papillary thyroid nuclear characteristics or PTC foci [46].
Among all subtypes of TCs, ATC is the most heterogeneous one. ATCs are characteristically very invasive tumors extending into the thyroid parenchyma and to the neighboring soft tissues and neck structures [47]. Clearly, ATCs are comprise of different features corresponding to the heterogeneity noted microscopically. ATC has monotonous paucicellular variant that is observed both at the macro and microscopic examination. ATC presents a wide range of differentiation, i.e., epithelioid cells, pleomorphic giant cells and spindle cells. ATC may simulate fibrosarcoma, angiosarcoma or rhabdomyosarcoma, solitary fibrous neoplasm, undifferentiated pleomorphic sarcoma, morphologically [48,49].
Medullary Thyroid Carcinoma (MTC)
MTC is a malignant tumour that develops in thyroid parafollicular or C cells. The primary secretory component of MTC is calcitonin, a biomarker that is both specific and highly sensitive and is produced by both healthy and malignant C cells. Neoplastic C cells also yield the Carcinoembryonic Antigen (CEA). Typically, these molecules are employed as markers for MTC case prognosis, diagnosis, and follow-up [50].
A subset of individuals who experienced both MTC and PTC simultaneously showed a significant incidence of known familial MTC. In other similar cases, co-expression of calcitonin and thyroglobulin was observed at both the protein and mRNA levels in the tumour cells, suggesting the possibility of their shared origin from stem cell differentiation. Nevertheless, this theory has not yet been authenticated together with the validation of the embryologic pathway [6,51-55].
Systemic Treatments for Thyroid Malignancy
Radioactive iodine (RAI) in low-risk thyroid carcinoma
The choice for RAI therapy and optimum quantity are related to the goals of treatment by use of classifications as under:
a) Remainder ablation occurs when normal thyroid tissue is “ablated” during treatment to allow for long-term monitoring and scan-directed staging.
b) Adjuvant therapy is used to treat cases when there is visible residual cancer or normal and malignant tissue;
c) Treatment when individuals are diagnosed with recurrent or residual thyroid cancer [56].
A “risk adapted” methodology has been encouraged for cases with low/intermediate-risk thyroid carcinoma to define the cases to be managed and to notify a quantity plan for treatment. This individualized tactic reflects the pathology risk, magnitude and entirety of surgical procedure along with response to procedure, established on Tg levels on levothyroxine and imaging 2-3 months post technique [57,58]. Cases of lesser risk TCs, (lymph nodes<5 and all are <2 mm), are not normally suggested for RAI, established on research displaying negative survival advantage or decline in residual/relapsing illness following RAI, principally if they have attained outstanding response considerations post-surgery [59- 61]. Opinions exist that are in contradiction of this conventional approach, referring to uncommon cases of identifying distant me tastases regardless of low post-operative Tg level [62]. This is particularly relevant in cases where there is a fantastic response, but the neoplasms have traits associated with intermediate to high-risk pathology. In such instances, the risk-adaptive technique may have a greater impact on the RAI dose provided than the decision to treat or not to treat. Lastly, it is not obvious yet whether specific genomic characteristics of thyroid carcinomas should affect the RAI usage [57].
In case of low to intermediate risk neoplasm with outstanding or uncertain surgical outcome, if RAI is given for remnant ablation or as adjuvant therapy, numerous randomized prospective research upkeep the practice of lesser dose (30mCi) instead of higher dose (100mCi) [63,64]. As per such data and consequent evidence-based guidelines, the RAI usage rate and the dosages employed in lowrisk thyroid carcinoma cases is declining. The California Cancer Registry was assessed by Park, et al. which revealed that the use of RAI reduced for cases with localized disease from 55% in 1999 to 30% in 2015 [65]. Complications due to RAI contribute to worse quality of life [66]. Many patients who had RAI never felt they had a choice, emphasizing the value of patient and healthcare practitioner collaboration in decision-making [57].
Systemic therapy in progressive metastatic differentiated thyroid carcinoma
Several patients of Differentiated Thyroid Cancer (DTC) with distant metastases continue experience chronic sickness years after surgery and RAI [67-69]. A subcategory of this cluster progresses over time, together with some that turn out to be swiftly progressive even with RAI [70,71]. The principal treatment mode for cases with advanced or huge extensive metastases previously was to give additional and greater RAI doses. Some patients might be benefitted yet, certain tumors carry on growing or no longer display iodine acceptance. Such a group of patients, along with those who progress <1 year post-treatment or do not respond to very high cumulative RAI dosages are regarded having RAIR (radio-iodine refractory) disease. In the cases with RAIR thyroid cancers disease, progression rate is high which is established on diminutive Tg or radiographic doubling times, large neoplasm sizes, poorly differentiated histology or indications. In such cases, systemic treatments are suggested [72,73].
Two official FDA-approved therapies with explicit signs for treatment of patients with RAIR thyroid carcinoma are multikinase inhibitors sorafenib and Lenvatinib [74]. There is lack of statistics on enhanced overall survival yet, In a prospective, randomised, crossover, phase III clinical trial, lenvatinib [76,77] and sorafenib [75] were associated with median Progression-free Survival (PFS) of 18.3 months vs. 3.6 months with placebo and 10.8 months vs. 5.8 months with placebo, respectively. There has been development of more targeted therapies due to advancement in the previous times in describing the molecular/genomic features of thyroid carcinoma. Some targeted medicines have FDA approval for tumor-indifferent usage based on the target’s or a biomarker’s expression. Rarely can rearranging the Neurotrophic Tropomyosin Receptor Kinase (NTRK) gene cause thyroid cancer. The selective inhibitors of TRK kinases are Larotrectinib and Entrectinib that can be employed in thyroid malignant cases concealing mutations or reordering in the NTRK genes [78-80]. Pembrolizumab is the anti-PD-1 monoclonal antibody which is official for neoplasms with greater microsatellite variability or greater PDL-1 ligand expression; it was studied in thyroid malignancy. Spartalizumab is the PD1 inhibitor which showed activity in ATC [81,82].
Cases of BRAFV600E-mutated DTC have displayed promising results with BRAFV600E inhibitors studies i.e. vemurafenib was linked with medial PFS of 18.2 months in RAIR PTC cases [49]. Moreover, for BRAFV600E-mutated ATC, the blend therapy of BRAFV600E and MEK inhibitors dabrafenib and trametinib are FDA-approved. Redifferentiation RAS or BRAFV600E-mutated RAIR DTC so for regaining RAI-sensitivity employing MEK inhibitors (RAS-mutated) or BRAFV600 ± MEK inhibitor (BRAFV600-mutated) are under study for particular cases of thyroid carcinoma [83-85]. There are two second-generation inhibitors of RET namely Selpercatinib [86] and Pralsatinib [87] that were FDA approved for RET-mutated and RET-rearranged malignancies, including PTC and MTC [57].
Conclusion
TCs heterogeneity is a clinical challenge, particularly the intratumor inconsistency. The researchers conducted until now reveal a broad range of variations, demonstrating “drivers” and “passengers” that may be employed as molecular targets, in targeted treatments. There is also some conflicting data indicating the sub clonality of main TC mutations and the co-occurrence of diverse mutations within a sole tumor. Drug development effective for every discrete tumor clone is not possible due to the related toxicity. Hence, the ideal current methodology seems to be focusing on the principal driving mutation existing in the entire tumor cell population. Inhibition of the main signaling pathways causing neoplastic transformation and cancer progression may be a further approach. The greater intratumor heterogeneity of TCs also gives rise to drug resistance. Besides, at present, majority available chemotherapeutic agents work by slaying actively dividing tumor cells.
Pathologists should be conversant with the many heterogeneity stages in order to develop a clinically relevant diagnosis and collect high-quality material for advanced molecular research, keeping in mind the complications of inter- and intratumor heterogeneity. This will help in stratifying patients risks appropriately, choose markers for more efficacious therapeutic considerations and get individualized therapy as the standard management for malignant cases.
References
- Seib CD, Sosa JA (2019) Evolving understanding of the epidemiology of thyroid cancer. Endocrinology and Metabolism Clinics. 48(1): 23-35.
- Noone AM, Kathleen AC, Sean FA (2017) Cancer Incidence and Survival Trends by Subtype Using Data from the Surveillance Epidemiology and End Results Program, 1992–2013 Cancer Incidence and Survival Trends by Subtype, 1992–2013. Cancer Epidemiology, Biomarkers & Prevention 26(4): 632-641.
- Lee K (2021) Thyroid cancer, in StatPearls [Internet]. Stat Pearls Publishing.
- Bai Y, Kakudo K, Jung CK (2020) Updates in the pathologic classification of thyroid neoplasms: a review of the world health organization classification. Endocrinology and Metabolism. 35(4): 696.
- Dettmer MS (2015) Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocrine-related cancer 22(3): 419-429.
- Chmielik E, Dagmara R, Malgorzata OW, Michal J, Jolanta K, et al. (2018 ) Heterogeneity of Thyroid Cancer. Pathobiology 85(1-2): 117-129.
- Nowell PC, (1976) The Clonal Evolution of Tumor Cell Populations: Acquired genetic lability permits stepwise selection of variant sublines and underlies tumor progression. Science 194(4260): 23-28.
- Prete A, Souza PB, Simona C, Marina M, Nicole N, et al. (2020) Update on fundamental mechanisms of thyroid cancer. Frontiers in Endocrinology 11: 102.
- Takano T (2014) Fetal cell carcinogenesis of the thyroid: A modified theory based on recent evidence [My Opinion]. Endocrine Journal EJ13-0517.
- Zarkesh M, Azita ZV, Fereidoun A, Forough F, Maziar MA, et al. (2018) Altered epigenetic mechanisms in thyroid cancer subtypes. Molecular diagnosis & therapy 22(1): 41-56.
- Nikiforov YE, Nikiforova MN (2011) Molecular genetics and diagnosis of thyroid cancer. Nature Reviews Endocrinology 7(10): 569-580.
- Fagin J (2004) How thyroid tumors start and why it matters: kinase mutants as targets for solid cancer pharmacotherapy. Journal of Endocrinology 183(2): 249-256.
- Khatami F, Tavangar SM (2018) A review of driver genetic alterations in thyroid cancers. Iranian Journal of Pathology 13(2): 125.
- Carling T (2014) Udelsman RJArom. Thyroid Cancer 65: 125-137.
- Agrawal N (2014) Integrated genomic characterization of papillary thyroid carcinoma. Cell 159(3): 676-690.
- Yarchoan M, LiVolsi VA, Brose MS (2014) BRAF mutation and thyroid cancer recurrence. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 33(1): 7-8.
- Raman P, Koenig RJ (2014) Pax-8–PPAR-γ fusion protein in thyroid carcinoma. Nature Reviews Endocrinology 10(10): 616-623.
- Howell GM, SPHodak SP, Yip L (2013) RAS mutations in thyroid cancer. The oncologist 18(8): 926-932.
- Nikiforova MN, Abigali W, Somak R, MaryBD, Yuri N, et al. (2013) Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. The Journal of Clinical Endocrinology & Metabolism. 98(11): E1852-E1860.
- Arighi E, Borrello MG, Sariola H (2005) RET tyrosine kinase signaling in development and cancer. Cytokine & growth factor reviews. 16(4-5): 441-467.
- Landa I, Ian G, Timothy AC, Norisato M, MichikoM, et al. (2013) Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. The Journal of Clinical Endocrinology & Metabolism 98(9): E1562-E1566.
- Malchoff CD, Malchoff DM (2006) Familial nonmedullary thyroid carcinoma. Cancer Control 13(2): 106-110.
- Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA: a cancer journal for clinicians. 65(1): 5-29.
- Fiore M, Gea OC, Rosario C, Antonino B, Pietro Z, et al. (2019) Role of emerging environmental risk factors in thyroid cancer: a brief review. International journal of environmental research and public health 16(7): 1185.
- MAZZAFERRI EL, (1999) An overview of the management of papillary and follicular thyroid carcinoma. Thyroid 9(5): 421-427.
- Pozdeyev N (2018) Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clinical Cancer Research 24(13): 3059-3068.
- Schopper HK (2017) Single thyroid tumour showing multiple differentiated morphological patterns and intramorphological molecular genetic heterogeneity. Journal of clinical pathology. 70(2): 116-119.
- Fugazzola L (2020) Intratumoral genetic heterogeneity in papillary thyroid cancer: occurrence and clinical significance. Cancers 12(2): 383.
- Colombo C (2019) Impact of mutation density and heterogeneity on papillary thyroid cancer clinical features and remission probability. Thyroid 29(2): 237-251.
- Baloch Z, LiVolsi VA, Tondon R (2013) Aggressive variants of follicular cell derived thyroid carcinoma; the so called ‘real thyroid carcinomas’. Journal of clinical pathology. 66(9): 733-743.
- Chen JH (2011) Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Modern Pathology 24(5): 739-749.
- Lubitz CC (2014) Hobnail variant of papillary thyroid carcinoma: an institutional case series and molecular profile. Thyroid 24(6): 958-965.
- Chung YJ (2013) Histomorphological factors in the risk prediction of lymph node metastasis in papillary thyroid carcinoma. Histopathology 62(4): 578-588.
- Kuhn E (2012) Different clonal origin of bilateral papillary thyroid carcinoma, with a review of the literature. Endocrine pathology 23(2): 101-107.
- Pfeifer A (2013) Molecular differential diagnosis of follicular thyroid carcinoma and adenoma based on gene expression profiling by using formalin-fixed paraffin-embedded tissues. BMC medical genomics 6(1): 1-10.
- Pstrąg N (2018) Thyroid cancers of follicular origin in a genomic light: in-depth overview of common and unique molecular marker candidates. Molecular Cancer 17(1): 1-17.
- Nikiforova MN (2003) RAS point mutations and PAX8-PPARγ rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. The Journal of Clinical Endocrinology & Metabolism 88(5): 2318-2326.
- Jung SH (2016) Mutational burdens and evolutionary ages of thyroid follicular adenoma are comparable to those of follicular carcinoma. Oncotarget 7(43): 69638.
- Yoo SK (2016) Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS genetics 12(8): e1006239.
- Hou P (2007) Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clinical cancer research 13(4): 1161-1170.
- Da Silva (2011) Tumor heterogeneity in a follicular carcinoma of thyroid: A study by comparative genomic hybridization. Endocrine pathology. 22(2): 103-107.
- Mete O, Asa SL (2016) Endocrine Pathology with Online Resource. Cambridge University Press, USA.
- Gibson WJ (2017) Genomic heterogeneity and exceptional response to dual pathway inhibition in anaplastic thyroid cancer. Clinical Cancer Research 23(9): 2367-2373.
- Romei C (2018) Clinical pathological and genetic features of anaplastic and poorly differentiated thyroid cancer: A single institute experience. Oncology letters 15(6): 9174-9182.
- Yakushina VD, LernerLV, LavrovAV (2018) Gene fusions in thyroid cancer. Thyroid 28(2): 158-167.
- Landa I (2016) Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. The Journal of clinical investigation 126(3): 1052-1066.
- Lam AK (2021) Anaplastic thyroid Abe carcinoma: Updates on WHO classification, clinicopathological features and staging. Histol Histopathol 36(3): 239-248.
- Pozdeyev N ( 2020) Molecular therapeutics for anaplastic thyroid cancer. in Seminars in cancer biology. Elsevier.
- Luo H (2021) Characterizing dedifferentiation of thyroid cancer by integrated analysis. Science Advances 7(31):
- Ceolin L (2019) Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocrine-related cancer 26(9): R499-R518.
- Giovanella L (2021) Procalcitonin as an alternative tumor marker of medullary thyroid carcinoma. The Journal of Clinical Endocrinology & Metabolism 106(12): 3634-3643.
- Oczko-Wojciechowska M (2015) The prevalence of somatic RAS mutations in medullary thyroid cancer-a Polish population study. Endokrynologia Polska 66(2): 121-125.
- Elisei R (2019) Twenty-five years experience on RET genetic screening on hereditary MTC: an update on the prevalence of germline RET mutations. Genes 10(9):
- Rossi ED (2020) The diagnosis of hyalinizing trabecular tumor: a difficult and controversial thyroid entity. Head and Neck Pathology 14(3): 778-784.
- Nikiforova MN (2019) GLIS rearrangement is a genomic hallmark of hyalinizing trabecular tumor of the thyroid gland. Thyroid 29(2): 161-173.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, et al. (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1): 1-133.
- Nabhan F, Dedhia PH, Ringel MD (2021) Thyroid cancer, recent advances in diagnosis and therapy. International Journal of Cancer 149(5): 984-992.
- Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, et al. (2018) NCCN guidelines insights: thyroid carcinoma, version 2.2018. Journal of the National Comprehensive Cancer Network 16(12): 1429-1440.
- Nixon IJ, Ganly Ian, Patel SG, Palmer FL, Lorenzo MMD, et al. (2013) The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on Memorial Sloan-Kettering Cancer Center risk group stratification. Thyroid 23(6): 683-694.
- Lamartina L, Leboulleux S, Terroir M, Hartl D, Schlumberger M, et al. (2019) An update on the management of low-risk differentiated thyroid cancer. Endocrine-Related Cancer 26(11): R597-R610.
- Matrone A, Gamble C, Piaggi P, Viola D, Giani C, et al. (2017) Postoperative thyroglobulin and neck ultrasound in the risk restratification and decision to perform 131I ablation. The Journal of Clinical Endocrinology & Metabolism 102(3): 893-902.
- Giovanella L, Avram AM, Clerc J, Hindié E, Taïeb D, et al. (2018) Postoperative serum thyroglobulin and neck ultrasound to drive decisions about iodine-131 therapy in patients with differentiated thyroid carcinoma: an evidence-based strategy?. Eur J Nucl Med Mol Imaging 45: 2155-2158.
- Dehbi HM, Mallick U, Wadsley J, Newbold K, Harmer C, et al. (2019) Recurrence after low-dose radioiodine ablation and recombinant human thyroid-stimulating hormone for differentiated thyroid cancer (HiLo): long-term results of an open-label, non-inferiority randomised controlled trial. The Lancet Diabetes & Endocrinology 7(1): 44-51.
- Schlumberger M, Leboulleux S, Catargi B, Deandreis D, Zerdoud S, et al. (2018) Outcome after ablation in patients with low-risk thyroid cancer (ESTIMABL1): 5-year follow-up results of a randomised, phase 3, equivalence trial. The Lancet Diabetes & Endocrinology 6(8): 618-626.
- Park KW, Wu JX, Du L, Leung AM, Yeh MW, et al. (2018) Decreasing use of radioactive iodine for low-risk thyroid cancer in California, 1999 to 2015. The Journal of Clinical Endocrinology & Metabolism 103(3): 1095-1101.
- Goswami S, Peipert BJ, Mongelli MN, Kurumety SK, Helenowski IB, et al. (2019) Clinical factors associated with worse quality-of-life scores in United States thyroid cancer survivors. Surgery 166(1): 69-74.
- Grani G, Ramundo V, Verrienti A, Sponziello M, Durante C, et al. (2019) Thyroid hormone therapy in differentiated thyroid cancer. Endocrine 66(1): 43-50.
- Klubo-Gwiezdzinska J, Auh S, Gershengorn M (2019) Association of thyrotropin suppression with survival outcomes in patients with intermediate-and high-risk differentiated thyroid cancer. JAMA network open 2(2): e187754-e187754.
- Buffet C, et al. (2020) Redifferentiation of radioiodine-refractory thyroid cancers. Endocrine-Related Cancer 27(5): R113-R132.
- Rajan N, Khanal T, Ringel MD (2020) Progression and dormancy in metastatic thyroid cancer: concepts and clinical implications. Endocrine 70(1): 24-35.
- Fullmer T, Cabanillas ME, Zafereo M (2021) Novel therapeutics in radioactive iodine-resistant thyroid cancer. Front Endocrinol 12: 720723.
- Bible KC, Cote GJ, Demeure MJ, Elisei R, Jhiang S, et al. (2015) Correlative studies in clinical trials: a position statement from the International Thyroid Oncology Group. The Journal of Clinical Endocrinology & Metabolism 100(12): 4387-4395.
- Sabra MM, Sherman EJ, Tuttle RM (2017) Tumor volume doubling time of pulmonary metastases predicts overall survival and can guide the initiation of multikinase inhibitor therapy in patients with metastatic, follicular cell‐derived thyroid carcinoma. Cancer 123(15): 2955-2964.
- Naoum GE, Morkos M, Kim B, Arafat W (2018) Novel targeted therapies and immunotherapy for advanced thyroid cancers. Molecular cancer 17(1): 1-15.
- Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, et al. (2014) Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. The Lancet 384(9940): 319-328.
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, et al. (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New England Journal of Medicine 372(7): 621-630.
- Lorusso L, Cappagli V, Valerio L, Giani C, Viola D, et al. (2021) Thyroid cancers: From surgery to current and future systemic therapies through their molecular identities. International Journal of Molecular Sciences 22(6): 3117.
- Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, et al. (2018) Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. New England Journal of Medicine 378(8): 731-739.
- Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N,et al. (2018) Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol 19(5): 705-714.
- Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, et al. (2020) Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 21(2): 271-282.
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, et al. (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357(6349): 409-413.
- Capdevila J, Wirth LJ, Ernst T, Aix SP, Chia-Chi Lin, et al. (2020) PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol 38(23): 2620-2627.
- Ho AL, Grewal RK, Leboeuf R, Sherman EJ, et al. (2013) Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. New England Journal of Medicine 368(7): 623-632.
- Jaber T, Waguespack SG, Cabanillas ME, Elbanan M, Vu T, et al. (2018) Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. The Journal of Clinical Endocrinology & Metabolism 103(10): 3698-3705.
- Rothenberg SM, Daniels GH, Wirth LJ, Daniel GH, Palmer E (2015) Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib-response. Clin Can Res 21(24): 1028-1035.
- Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, et al. (20200 Efficacy of selpercatinib in RET-altered thyroid cancers. New England Journal of Medicine 383(9): 825-835.
- Markham A (2020) Pralsetinib: first approval. Drugs 80(17): 1865-1870.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...