Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Mini Review(ISSN: 2641-1725) 
Role of Chloroquine and Hydroxychloroquine in the Treatment of COVID 19 Volume 5 - Issue 2
Nisreen H Meiqal1, Salah M Bensaber1, Mousa I Jaeda1, Anton Hermann2 and Abdul M Gbaj1*
- 1Department of Medicinal Chemistry, Faculty of Pharmacy, University of Tripoli, Libya
- 2Department of Biosciences, University of Salzburg, Salzburg, Austria
Received: June 16, 2020; Published: June 23, 2020
*Corresponding author: Abdul M Gbaj, Associate Professor of Genetics and Biochemistry, Department of Medicinal Chemistry, Faculty of Pharmacy, University of Tripoli, Libya
DOI: 10.32474/LOJMS.2020.05.000209
Abbreviations: (WHO) World Health Organization; (FDA) Food and Drug Administration; (EMA) European Medicines Agency
Introduction
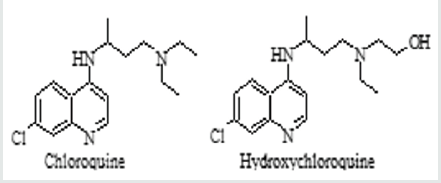
Chloroquine was discovered in 1934 by Hans Andersag (German scientist) while he was working for the Bayer Pharmaceutical Company, and this drug was initially called (Resochin) [1,2]. In 1945, the World Health Organization (WHO) recommended the use of chloroquine in the treatment of malaria, which was a deadly disease in many cases [3,4]. Both chloroquine and (safer analogue) with the chemical formula shown in Figure 1 are currently considered to be important drugs listed as anti-malaria agents according to the WHO, the US Food and Drug Administration (FDA), and the European Medicines Agency (EMA) [5,6]. Moreover, hydroxychloroquine is presently widely used to treat autoimmune diseases such as lupus and rheumatoid arthritis [7,8]. Both medications are considered safe drugs whose side effects are generally mild. However, their use must be subjected to strict rules, and self-treatment (without direct medical supervision) is not recommended due to their narrow therapeutic index [9,10].
Anti-viral activity
In vitro antiviral activity of chloroquine has been identified since the late sixties as scientists were able to inhibit the growth of many different viruses by chloroquine and hydroxychloroquine [11]. More recently, the ability of both drugs to inhibit the growth of viruses from the corona family (2004), as well as later the Ebola virus were identified [12-15]. Subsequently, several in vivo experiments in mice were conducted to test the efficacy in vitro of both drugs to inhibit the growth of viruses. The experiments suggested that the drugs were effective against both corona (OC43) [16,17] and influenza (A H5N1), but lack activity against the Ebola virus [18,19]. In addition, many clinical studies were conducted to test the ability of both chloroquine and hydroxychloroquine to inhibit the growth of viruses [20,21]. These clinical studies indicate that no severe viral infection can be treated with chloroquine or hydroxychloroquine although a modest effect of chloroquine in treating human infection with chronic hepatitis C was reported [22].
Antiviral activity against the emerging corona virus (SARS-CoV2)
After the emergence of the COVID-19 pandemic, many drugs and chemical compounds that inhibited viruses in the past were tested against SARS-CoV2 virus. Initially, in vitro investigation of chloroquine ‘s ability to inhibit the emerging corona virus (SARSCoV2) was reported in China (February 2020) indicating high activity [23,24]. These investigations were followed by several in vitro and clinical studies that aimed to demonstrate the ability of chloroquine and hydroxychloroquine to inhibit SARS-CoV2 virus in China and some other countries [25-27].
Based on the results of these studies, the following statements
can be concluded:
a. Chloroquine and hydroxychloroquine exhibit good in vitro
efficacy (anti SARS-CoV2) [25-27].
b. More in vivo studies are required to determine
the pharmacokinetic properties of Chloroquine and
hydroxychloroquine as possible anti SARS-CoV2 drugs
c. A variety of clinical studies are registered in various
countries to test chloroquine and hydroxychloroquine ability
to inhibit the emerging corona virus (SARS-CoV2) [28-31].
Accordingly, some specialized institutions in some countries
and international consortia started to include chloroquine
and hydroxychloroquine as part of their medical protocol to
treat COVID19 patients. However, this initiation is regarded
problematic due to the lack of proof of efficacy as well as fear of
unexpected clinical side effects [32-34].
d. Recently, clinical studies (multinational registry analysis)
of the use of chloroquine and hydroxychloroquine with or
without a macrolide (a class of natural products that consist of
a large macrocyclic lactone ring with one or more deoxy sugars
attached) for treatment of COVID-19 was reported based on
COVID 19 patients hospitalized in different countries. The
achieved results indicated lack of evidence of benefit of both
medications to effectively treat COVID 19 patients. In addition,
there was an associated increase of ventricular arrhythmias
and a greater hazard for in-hospital death with COVID-19 [35-
37].
e. Based on the findings listed in point (4), the FDA warned
against using of chloroquine and hydroxychloroquine outside
the medical framework, the WHO suspended their clinical
trials for safety reasons, and their use as part of the treatment
protocols were later stopped by the French government.
Conclusion
Chloroquine or its analogues appeared a hope for humanity in the treatment of the COVID-19 disease. Chloroquine is cheap and safe (if used under medical supervision), and early results of in vitro studies were promising. However, this initial achievement requires further investigation to verify the suitability and safety of their use. Currently, WHO does not recommend any specific antiretroviral drugs against SARS-CoV2, citing the lack of sufficient evidence to recommend any specific treatment that includes chloroquine or its analogues. To determine the efficacy of chloroquine or its analogues to treat COVID 19, it will be necessary to provide more financial support for the continuation of clinical trials.
Acknowledgement
The authors gratefully acknowledge the technical support and valuable suggestions obtained from Ms Amira Abdul Gbaj (Novelien Zone, Tripoli, Libya).
References
- Sá JM, Chong JL, Wellems TE (2011) Malaria drug resistance: new observations and developments.EssaysBiochem51: 137-160.
- Pou S, Winter RW, Nilsen A, Kelly JX, Li Y et al. (2012)Sontochin as a Guide to the Development of Drugs against Chloroquine-Resistant Malaria.AntimicrobAgents Chemother56(7): 3475-3480.
- Beausoleil E (1984) A review of present antimalaria activities in Africa.Bull.World Health Organ 62: 13-17.
- Okech BA, Existe A, Romain JR, Memnon G, Victor YS et al. (2015) Therapeutic Efficacy of Chloroquine for the Treatment of Uncomplicated Plasmodium falciparum in Haiti after Many Decades of its Use.AmJTropMedHyg92(3): 541-545.
- Mittal L, Zhang L, Feng R, Werth VP (2018) Antimalarial drug toxicities in patients with cutaneous lupus and dermatomyositis: a retrospective cohort study.JAmAcadDermatol78(1): 100-106.
- Browning DJ (2014) Pharmacology of Chloroquine and Hydroxychloroquine.Hydroxychloroquine.and ChloroquineRetinopathy4: 35-63.
- Smolen JS, LandewÃR, Breedveld FC, Buch M, Burmester G et al, (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update.AnnRheumDis73(3): 492-509.
- Urman A, Taklalsingh N, Sorrento C, McFarlane IM (2018) Inflammation beyond the Joints: Rheumatoid Arthritis and Cardiovascular Disease.ScifedJCardiol2(3): 1000019.
- Savage DE, Plotnik R, Wozniak RA (2020) Short-term high-dose hydroxychloroquine corneal toxicity.AmJOphthalmolCaseRep18: 100713.
- Motarjemizadeh Q, Aidenloo NS, Abbaszadeh M (2016) Detection of Hydroxychloroquine Retinal Toxicity by Automated Perimetry in 60 Rheumatoid Arthritis Patients With Normal Fundoscopic Findings.GlobJHealth Sci 8(3): 59-64.
- Dyall J, Gross R, Kindrachuk J, Johnson RF, Olinger GG et al. (2017) Middle East Respiratory Syndrome and Severe Acute Respiratory Syndrome: Current Therapeutic Options and Potential Targets for Novel Therapies.Drugs 77(18): 1935-1966.
- Zimmermann P, Curtis N (2020) Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology Clinical Features Diagnosis Treatment and Prevention Options in Children.PediatrInfectDisJ39(5): 355-368.
- Chan JFW, Lau SKP, To KKW, Cheng VCC, Woo PCY et al. (2015) Middle East Respiratory Syndrome Coronavirus: Another Zoonotic Betacoronavirus Causing SARS-Like Disease.ClinMicrobiolRev28(2): 465-522.
- Dhama K, Karthik K, Khandia R, Chakraborty S, Munjal A, et al. (2018) Advances in Designing and Developing VaccinesDrugs and Therapies to Counter Ebola Virus.Front Immunol9: 1803.
- Xiao JH, Rijal P, Schimanski L, Tharkeshwar AK, Wright E, et al. (2018) Characterization of Influenza Virus Pseudotyped with Ebolavirus Glycoprotein.JVirol92(4):e00941-e009417.
- Wang S, Zhang L, Zhang R, Chi X, Yang Z, (2018) Identification of two residues within the NS1 of H7N9 influenza A virus that critically affect the protein stability and function.VetRes 49: 98.
- Liu G, Park HS, Pyo HM, Liu Q, Zhou Y (2015) Influenza A Virus Panhandle Structure Is Directly Involved in RIG-I Activation and Interferon Induction.JVirol89(11): 6067-6079.
- Dhama K, Karthik K, Khandia R, Chakraborty S, Munjal A, et al. (2018) Advances in Designing and Developing Vaccines, Drugs, and Therapies to Counter Ebola Virus.Front Immunol 9: 1803.
- Dowall SD, Bosworth A, Watson R, Bewley K, Taylor I, et al. (2015) Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model.JGenVirol96(12): 3484-3492.
- Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R (2003) Effects of chloroquine on viral infections: an old drug against today's diseases.Lancet InfectDis3(11): 722-727.
- Delvecchio R, Higa LM, Pezzuto P, Valadão AL, Garcez PP, et al. (2016) Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models.Viruses8(12):322.
- Rodrigo C, Fernando SD (2020)Rajapakse SClinical evidence for repurposing chloroquine and hydroxychloroquine as antiviral agents: a systematic review.ClinMicrobiolInfect.
- Wang M, Cao R, Zhang L, Yang X, Liu J, et al. (2020)Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro.Cell Res30(3): 269-271.
- ŞİMŞEK YS, ÜNAL S (2020) Antiviral treatment of COVID-19.TurkJMedSci50(SI-1): 611-619.
- Felsenstein S, Herbert JA, McNamara PS, Hedrich CM (2020) COVID-19: Immunology and treatment options.ClinImmunol215: 108448.
- Roustit M, Guilhaumou R, Molimard M, Drici MD, et al. (2020) Chloroquine and hydroxychloroquine in the management of COVID-19: Much kerfuffle but little evidence. Therapie.
- Quiros RE, Biasiotto G, Magro P, Zanella I (2020) The possible mechanisms of action of 4-aminoquinolines chloroquine/hydroxychloroquineagainst Sars-Cov-2 infection (COVID-19): A role for iron homeostasis.PharmacolRes 2020; 158: 104904.
- Huang J, Song W, Huang H, Sun Q (2020) Pharmacological Therapeutics Targeting RNA-Dependent RNA PolymeraseProteinase and Spike Protein: From Mechanistic Studies to Clinical Trials for COVID-19.JClinMed9(4):1131.
- Abd El-Aziz TM, Stockand JD (2020) Recent progress and challenges in drug development against COVID-19 coronavirus SARS-CoV-2 - an update on the status.InfectGenetEvol83: 104327.
- Tobaiqy M, Qashqary M, Al Dahery S, Mujallad A, Hershan A, et al. Therapeutic management of patients with COVID-19: a systematic review2(3): 10006.
- Zhang J, Xie B, Hashimoto K (2020) Current status of potential therapeutic candidates for the COVID-19 crisis.BrainBehav.Immun 87: 59-73.
- Abd El-Aziz TM, Stockand JD (2020) Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status.InfectGenetEvol83: 104327.
- Yan Y, Shin WI, Pang YX, Meng Y, Lai J, et al. (2020) The First 75 Days of Novel Coronavirus (SARS-CoV-2) Outbreak: Recent Advances, Prevention, and Treatment.IntJEnvironResPublic Health17(7): 2323.
- Tobaiqy M, Qashqary M, Al Dahery S, Mujallad A, Hershan A, et al. (2020) Therapeutic management of patients with COVID-19: a systematic review2(3): 100061.
- Mehra MR, Desai SS, Ruschitzka F, Patel AN (2020) Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis.
- Roustit M, Guilhaumou R, Molimard M, Drici MD, Laporte S, et al. (2020) Chloroquine and hydroxychloroquine in the management of COVID-19: Much kerfuffle but little evidence.Therapie.
- Carrière F, Longhi S, Record M (2020) The endosomal lipid bis(monoacylglycero) phosphate as a potential key player in the mechanism of action of chloroquine against SARS-COV-2 and other enveloped viruses hijacking the endocytic pathway.Biochimie.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...