Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Research Article(ISSN: 2641-1725) 
Qualitative and Quantitative Analysis of Diphenylamine and N-nitrosodiphenylamine Using High Performance Thin Layer Chromatography Method Volume 3 - Issue 4
Namir Halilović1,2*, Azra Bašić-Halilović3,4, Raif Hadžić2, Igor Malešević2, Dražen Starčević2 and Miro Jurčević2
- 1Department of Chemistry, University of Sarajevo, Bosnia and Herzegovina
- 2Ammunition Surveillance Laboratory, Armed Forces of B&H, Bosnia and Herzegovina
- 3Faculty of Pharm and Health, University of Travnik, Bosnia and Herzegovina
- 4Institute for Biomedical Research and Diagnostics GENOM, Bosnia and Herzegovina
Received: September 26, 2019; Published: October 03, 2019
*Corresponding author: Namir Halilović, Department of Chemistry, University of Sarajevo, Bosnia and Herzegovina
DOI: 10.32474/LOJMS.2019.03.000169
Abstract
DPA and N-NODPA are used to maintain the stability of nitrocellulose propellants. In this paper, we examined the possibility of using High Performance Thin Layer Chromatography (HPTLC) for the qualitative and quantitative determination of DPA and N-NODPA, using a toluene as the carrier solution. 42% of all results showed difference in range up to ±0.01% (±8ng), and the other 58% showed full agreement of results at 254 nm. The results of the HPTLC in comparison with HPLC test have shown that this method can be used in the qualitative and quantitative analysis of single-base propellants, tested on propellants samples of ammunitions calibers of 20, 30, 100, 105, 122 and 125mm. With increasing of [%] DPA and N-NODPA we detected decreasing of released NOx gases from almost all tested calibers. The results obtained by the HPTLC method, in combination with other analytical methods such as the vacuum stability test, can be successfully used to predict the stability of propellants.
Keywords: Diphenylamine; N-nitrosodiphenylamine; HPTLC method; Propellant stability
General Information
Gunpowder consisting of nitrocellulose alone is termed singlebase gunpowder (SB), and those powders that contain nitroglycerine besides nitrocellulose are called double-base gunpowder (DB) [1].
In addition, DB propellants with picrite or nitro- guanidine added to the formulation are considered as a separate class and are called Triple Base (TB) propellants.
This part of the process is an unimolecular reaction and, according to the kinetic equation of Arrhenius, only the temperature is an influencing factor. The radicals formed during the first step of the reaction exponentially activate the oxidative degradation of NC, which may lead to an auto-catalytic degradation of the propellant. To avoid such an autocatalytic process, stabilizers are introduced in the propellant formulation, to catch the nitrogen-oxides (NOx) formed during the first part of the degradation reaction. By introducing stabilizers, the first part of the degradation is not influenced but the second part is broken down.
The stabilizers have the ability to response with nitrogen oxides by the formation of nitrosated and nitrated compounds. Typical stabilizers for propellants are displayed in [2] and for this work we focused on diphenylamine DPA and N-nitrosodiphenylamine NODPA alone, and in mixture with centralites and akardites.
The concentration of stabilizers in the propellant gives us important information about the actual grade of propellant decomposition provided that we know initial concentration of stabilizers.
According to the work of Marqueyrol, if the quantity of diphenylamine introduced into DB propellants reaches or exceeds 3-4%, an incompatibility problem occurs. Since 2-nitrodiphenylamine still possesses stabilizing properties and is compatible with NG, it can consequently be used in some double base propellants [3].
The elaboration of more recent stabilizers (centralite I, II, II, MNA, acardite I, II, III) completely compatible with NG and other nitroglycols, allows new DB and triple base propellants to be produced which may be better stabilized and may be stored for a longer period [4]. Stabilizers for doubla-base and nitroguanidine rocket propellants are often derivatives of urea (sometimes incombination with diphenylamine, DPA) [5].
The content of “effective stabilizer” is calculated from the contents of all initial stabilizers (diphenylamine, 2-nitrodiphenylamine, ethyl centralite, methyl centralite, akardite-II, p-nitro-N-methylaniline, resorcinol -except if they are used as surface moderators) and the content of N-nitrosodiphenylamineas follows:
For propellants without diphenylamine as well as for propellants with diphenylamine and other stabilizers:
Effective stabilizer = Ʃ (contents of initial stabilizers)
For propellants with diphenylamine only:
Effective stabilizer = content of diphenylamine + 0.85 N-nitrosodiphenylamine
Percentage Effective Stabilizer is the amount of effective stabilizer found, expressed as a percentage by weight of the propellant sample [6].
The article [2,7] explains the formation of nitric acid and nitric oxide in gunpowder in details, and the final result of the reactions is an increase in ambient temperature, which could theoretically be one of the causes of gunpowder inflammation. Stabilizers prevent the described occurrence of nitrogen compounds, nitric acid and nitrous oxides.
A property stabilized NC propellant is stable for a relatively long period of time (20-50 years) at normal storage conditions [8].
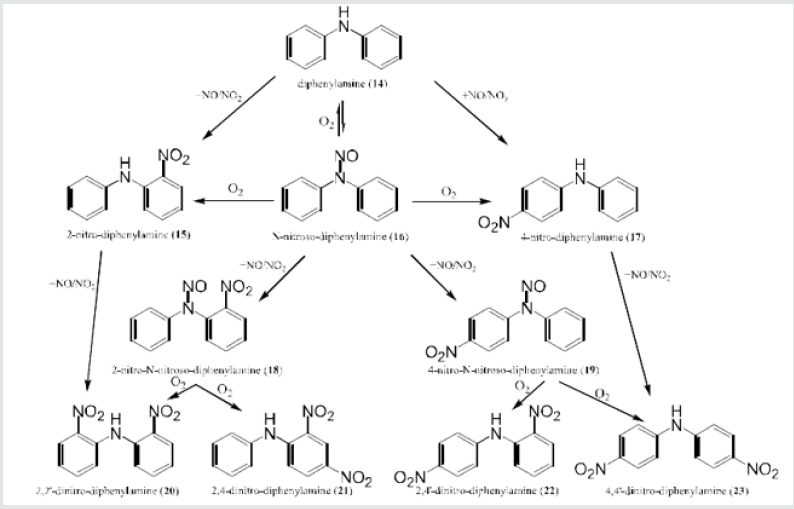
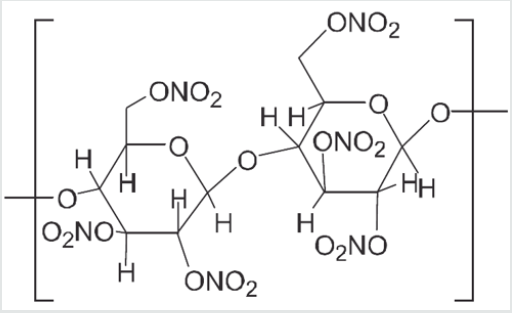
Diphenylamine fixes nitrogen oxides by a series of nitrosation and nitration reactions or transnitrations, which are both sequential and competitive (Figure 1) [3].
A study of the different nitro derivatives of diphenylamine gives precise information about the ageing state of a propellant and of ammunition, respectively. Chemical analysis using HPTLC gives a fingerprint of the ageing of the propellant.
HPTLC for Propellants Analysis
Currently, most planar chromatography is based on the thinlayer technique, which is faster, has better resolution, and is more sensitive than its paper chromatography equivalent [9].
Today, such techniques have largely been replaced by LC methods, which are readily automated and faster. Thin-layer chromatography has found widespread use in clinical laboratories is the backbone of many biochemical and biological studies. It also finds extensive use in industrial laboratories [10].
Typical thin-layer separations are performed on a glass plate coated with a thin and adherent layer of finely particles; this layer constitutes the stationary phase. Mobile phases are also similar to those found in HPLC [9].
For a better understanding of a thin layer chromatographic separation for analysis of propellants, we described it and compare its results with Vacuum Stability Test (VST) and High Performance Liquid Chromatography (HPLC) which are standard methods according NATO AOP 48 and STANAG 4556.
Sample preparation
For a chromatographic separation the sample must meet several requirements to obtain good results. According to sample, it is necessary to select suitable solvents of high purity. After full dissolving of sample in organic solvent, it is necessary to add pure water. After this step, NC from solution will precipitate.
We use pure water in this step, because NC is insoluble in water, which allows its preparation, stabilization, and transport by quenching, using the appropriate quantity of water. After it, we used 0,25μm filter paper, or in same cases we can use 0,45μm.
The solubility of NC according to [11] as a function of the nitrogen content give us information that we can use acetone in purpose of sample preparation. In our work, because of similarity, we used acetonitrile. Sample application is perhaps the most critical aspect of thin-layer chromatography, particularly for quantitative measurements. Usually, the sample, as a 0.01% to 0.1% solution, is applied as a spot 1 to 2cm from the edge of the plate. For best separation efficiency, the spot should have a minimal diameter - about 5mm for qualitative work and smaller for quantitative analysis.
For dilute solutions, three or four repetitive applications are used, with drying between [9].
Sample application and developing a chromatogram
The aim of a chromatographic separation determines how the sample should be applied to the TLC plate or sheet. The most frequent technique still is application with a glass capillary as spot or short streak.
In our laboratory in qualitative/quantitative evaluation we use Linomat 5 sample applicator for automatic spotting of samples on HPTLC Silica gel plate. We use plate with dimension 10 x 20cm.
After application allow the solvent of the samples to evaporate completely (about 10 minutes) or blow with cold or hot air. Development of a chromatogram should never start before the solvent of the applied samples is evaporated completely [12].
Qualitative detection
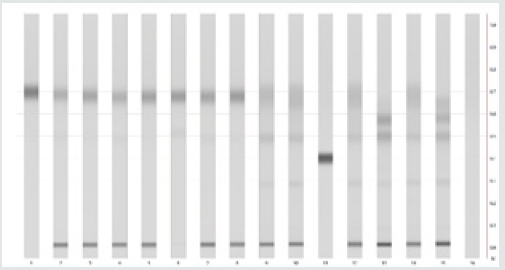
Qualitative detection of the stabilizer in gunpowder can be done by comparing the positions of the corresponding peaks in the developed HPTLC or TLC plate. Plate looks like in Figure 2, respectively in Figures 1,3 & 4.
In addition, there is always the possibility that two quite different solutes may exhibit identical or nearly identical Rf values under a given set of conditions.
Quantitative detection
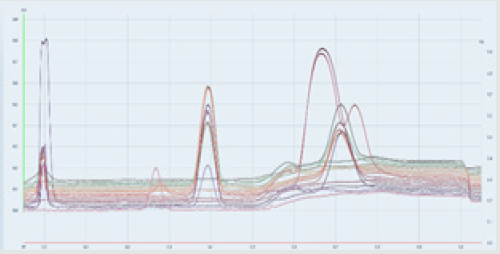
There are several advanced generations of scanners. In new generation of scanners signal to noise ratio was improved, spectral range extended: 190 - 900nm.
A semi quantitative estimate of the amount of a component present can be obtained by comparing the area of a spot with that of a standard. More accurate results can be obtained by scraping the spot from the plate, extracting the analyte from the stationaryphase solid, and measuring the analyte by a suitable physical or chemical method. In a third method, a scanning densitometer can be used to measure fluorescence or absorption of the spot [9].
For quantitative detection in this work with HPTLC plates automatic scanner by CAMAG are used.
Already authors [5] examined the use of the chromatographic method for analysis of double-base rocket propellants with centralit like main stabilizer, so in this article we choose to make research on single-base propellants with other main stabilizers.
Discussion
Chemicals
For developing HPTLC method in purpose of analytical tests on propellants we used next equipment and chemicals:
- CAMAG LINOMAT 5 (automatic sample applicator)
- CAMAG TLC SCANNER (quantitative detector)
- LINOMAT SYRINGE 695.0014 - 100μL
- Sterilized syringe – 2ml
- Syringe filters, RC 0,2μm
- Short Thread Vial – 1,5ml
- Pure N2(g)
- Diphenylamine 99%, SIGMA-ALDRICH
- N-nitrosodiphenylamine ≥97%, SIGMA-ALDRICH
- Sartorius electronical scale, class 1
- Acetone 99,8%, Fisher Scientific, U.K.
- Toluene 99,9%, Fisher Scientific, U.K.
- Acetonitrile 99,8%, CARLO ERBA, France
- Distilled water
- HPTLC Silica gel 60 F254(20x10cm)
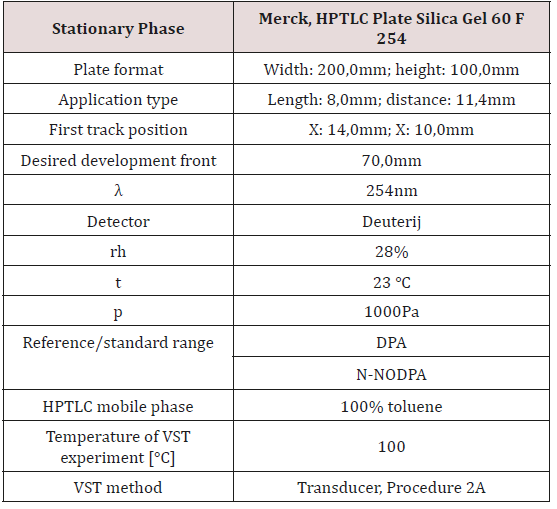
Test conditions
Procedure
According to instruction given in text above, we prepare samples of propellants, then we developed HPTLC plates, one of them in purpose of stabilizer detection, qualitative detection, and the other in purpose of quantitative detection of DPA and N-NODPA.
For qualitative analysis we tested a lot of different radio of solvents for developing a chromatograph, and in results we will show one of the best. For quantitative analysis of DPA and N-NODPA we tested also a lot of different solvents with different ratio, and we will present one of them with very high R2 factor. On the HPTLC plate we use eight spots for application of reference solution of stabilizers DPA and N-NODPA. The other eight spots we use for application of propellants samples. It is important to mention that we used two spots each time for one reference or sample, and for calculation CAMAG software used average value. So, on one plate we can examine four propellants sample.
Results and Discussion
Qualitative detection
For this experiment we get clear result picture/graphic. After application of prepared propellants samples, we developed chromatograph in solvent mixture of toluene and acetone and result in form of picture is showed below.
Next step is determinate right position, Rf, of stabilizers standard CI and CII, and on comparison principle found witch kind of stabilizer is in propellant sample. For that purpose, we found Rf values for standard solutions showed in Table 1 below.
In situation that you can`t clearly decide from Figure 5 which stabilizer is present in propellant, then you can use more clearly way, but in the other hand, more slowly way of stabilizer determination. It is one kind of results graphic show, which you can use in the CAMAG software.
The results obtained are shown in the Tables 2-4 (Row Detected stabilizers).
Table 2: Rf (found) values for some stabilizers after developed HPTLC/TLC plate in solvent mixture of toluene and acetone.
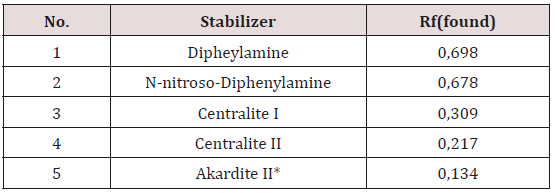
*Akardite II or AII (1-Methyl-3,3-Diphenylurea)
Quantitative detection
For this experiment, according to instruction from HPLC operators [13], we make standard solutions in acetonitrile for DPA and N-NODPA. We propose range 0.2-0.8% and 0.8-1.4% or higher, depend of propellants sample.
Reason for this kind of range is because of facts that content of DPA in propellants are usually about 2%, and in some ammunition is even more, up to 3-4% by author Marqueyrol presented in [3].
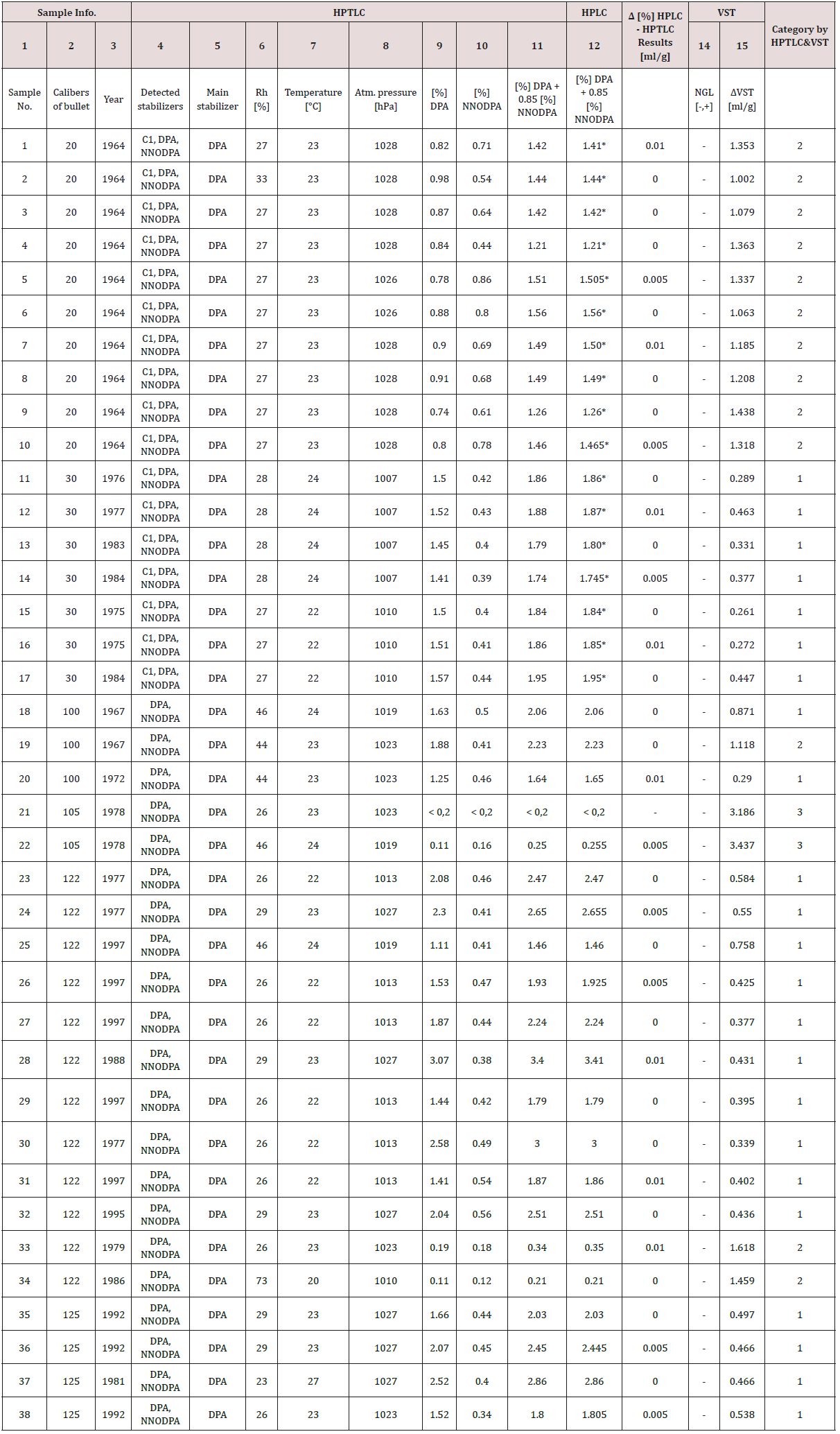
The Table 4 consist results from 38 single-based propellants samples which selected like representative in our researches. We use samples of propellants from 6 different calibers of ammunition: 20, 30, 100, 105, 122 and 125mm. These samples are from different years:
- 1964 – 10 samples
- 1967 – 2 samples
- 1972 – 1 sample
- 1975 – 2 samples
- 1976 – 1 sample
- 1977 – 4 samples
- 1978 – 2 samples
- 1979 – 1 sample
- 1981 – 1 sample
- 1983 – 1 sample
- 1984 – 2 samples
- 1986 – 1 sample
- 1988 – 1 sample
- 1992 – 3 sample
- 1995 – 1 sample
- 1997 – 5 samples
We choose 10 samples from 1964 because we want examine stability of them and made some conclusions about it.
First 17 samples from Table 4 consist DPA, N-NODPA and Centralite I, and the others consist DPA and N-NODPA. Because of amount of concentration, we chose DPA like main stabilizer. We can mention that there is a big similarity between HPTLC (R2 factors in our experiments are above 0.9975) and HPLC results. With increase contents of stabilizer, generally we can mention decrease in released gas on VST method. For better view see Table 4 and compare column 11 and 15.
In Table 3 the best view is on caliber 20mm. In this situation we have same year of ammunition production and in this samples we also have Centralite 1 in contest of them. We can mention that with increasing of [%] DPA + 0.85 [%] N-NODPA we detected decreasing of released NOx gases from samples and this fact is so important from our experiments. To be more specified, some differences are present, but we can mention that these samples contain Centralie I, and it is lazy stabilizer, so according to this minor disagreements were expected.
Table 3: Results comparison according to increase [%] DPA + 0.85 [%] NNODPA.
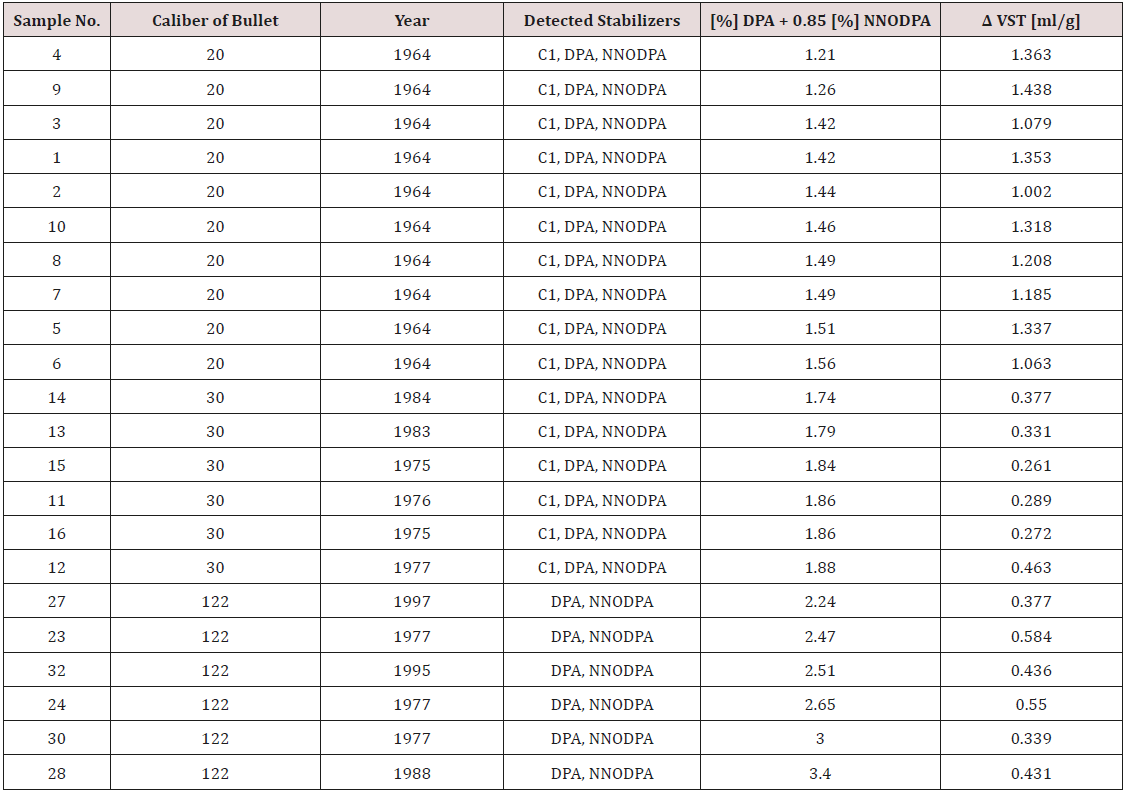
*For propellants witch contain C1, DPA and N-NODPA effective stabilizer calculates like Ʃ contents of initial stabilizers, but in this table we do not use this formula because we want make conclusions based only on content od DPA and N-NODPA.
On caliber 30mm we mention the same situation like described above, only difference is in sample 12, and this disagreement we can attribute to year of sample production. If we exclude sample 16, the sample 12 is the oldest from group of 30mm ammunition caliber [14,15].
In group of 122mm ammunition caliber we also have decreasing pressure by VST in coordination with increasing amount of stabilizers. Some differences are in sample 122, and this fact we can explain with information that manufacturer of this sample is unknown and history of storage is also unknown for us.
According to Bosnian National Standards from field of stability of propellants witch are in coordination with NATO standards AOP-48 and STANAG 4556, we combine HPTLC and VST results to create category of propellants stability. We tested samples on nitroglycerine content (14-NGL column in Table 4).
In our case we prepare 2.000g (±0.080g) samples of propellants for HPTLC and HPLC according to instructions described in text above [16-18]. After that step, we use 4μl of sample for qualitative experiment and other 4μl for quantitative experiment. Results in Table 4 in column 4 are results for qualitative determination of stabilizers based on procedure showed on Figures 6,7 & Table 2.
Final, Table 4 show comparison of results on two quantitative methods, HPTLC and HPLC and additionally VST method in purpose of stability prediction.
Analysis on HPLC method done in institute GENOM by second author of this article, and for mobile phase we use ratio of acetonitrile, methanol and water. Analysis on both methods are made on same days in two different laboratories, and the similarity of results is very good (see Table 4, column 13).
For categorization [2] we need percentage of main stabilizers, which are calculated according to AOP 48 mentioned above in article. In eight samples: 5, 10, 14, 22, 24, 26, 36, 38 we have some small difference in results, 0.005 %, and in the other eight samples: 1, 7, 12, 16, 20, 28, 31, 33 difference is 0.01%. According to this results, 42% of all results showed difference in range up to 0.01%, and the other 58% showed agreement of results. We can explain it by individual mistakes from process of sample preparation, which include mistake by scale, the process of dilution, or in process of chamber development etc. To be sure about this explanation, we make some samples in laboratory and use the same for both analysis: HPTLC and HPLC. Results obtained from this procedure showed better similarity, then the others. The other fact is type of method, HPLC is more sensitive method with better properties, but according to results, HPLC is also very useful method for purpose of propellants analysis.
It is important to mention that in this article we present results in form of percentage because for stability categorization we need it, and it means:
±8ng → ±0.01%
Conclusion
Results obtained from HPTLC method are useful in purpose of qualitative and quantitative analysis of propellants samples. Qualitative analysis of propellants samples on HPTLC method can be used successfully for detection of main stabilizer based on comparison with references.
DPA and N-NODPA can be determined qualitatively by HPTLC using a mixture of toluene and acetone as carrier solvent on 254nm.
This similarity of quantitative results are very good and it should be noted that in our case we used different analytical devices with different analytical performances, HPTLC and HPLC. 42% of all results showed difference in range up to ±0.01% (±8ng), and the other 58% showed full agreement of results. According to results and rules of propellants categorization, HPTLC method can be used in purpose of quantitative analysis of propellants stabilizers on 254nm.
Toluene can be used like mobile phase in purpose of HPTLC plate development for analysis content of diphenylamine and N-nitrosodiphenylamine alone, or in mixture with 1,3-diethyl-1,3- diphenylurea (CI),1,3-dimethyl-1,3-diphenylurea (CII), 1-methyl- 3,3-diphenylurea (AII). Quantitative analyzes of propellants from the HPTLC method can be used to determine and predict the safety status of propellants in combination with Vacuum Stability Test.
References
- EEON (1994) Characterization of smokeless gunpowder by means of diphenylamine stabilizer and its nitrated derivatives. Analytica Chimica Acta 288(1-2): 57-69.
- Halilović N, Hadžić R, Malešević I, Jurčević M, Starčević D (2019) Application of thin layer chromatography for qualitative analysis of gunpowder in purpose of life prediction of ammunition. International Journal of Biosensors & Bioelectronics 5(1): 4-12.
- Folly P, Mader P (2004) EXPLOSIVES - Propellant Chemistry, No. 6 edition. Schweizerische Chemische Gesellschaft pp. 374-382.
- Quinchon JTMNJ (1986) Les poudres, propergols et explosifs: 3. Les poudres pour armes, Paris: Technique et Documentation (Lavoisier).
- Sanja Matečić Mušanić MSRČ (2003) The Applicability of Chromatographic Methods in the Investigation of Ageing Processes in Double Base Rocket Propellants. Central European Journal of Energetic Materials 10(2): 245-262.
- (2008) AOP-48, EXPLOSIVES, Nitrocellulose-Based Propellants, Stability Test Procedures and Requirements Using Stabilizer Depletion. North Atlantic Treaty Organization p. 48.
- Cho KH (2010) A study on the self-life estimation of the propellant KM10. Journal of the Korean 5(11): 1735-1740.
- Bergens, Danielsson R (1995) Decomposition of diphenylamine in nitrocellulose based propellants-I. optimazation of a numerical model to concentration-Time data for diphenylamine and its primary degradation products determined by liquid chromatography with dual-amperometric detection. Pergamon Elsevier Science Ltd pp. 171-183.
- Skoog DA, Holler FJ, Crouch SR (2007) Principles of Instrumental Analysis. Belmont. Thomson Higher Education.
- Sherma J, Fried B (2003) Handbook of Thin-Layer Chromatography. New York, USA.
- Champetier G, Les dérivés cellulosiques, Dunod Ed (1954) Paris, p. 260.
- Macherey N (2018) Basic principles of TLC. p. 268-312.
- Sarbach I (2018) HPTLC training [Interview]. p. 16-19.
- Lopez Lopez M, Bravo JC, Garcia-Ruiz C, Torre M (2013) Diphenyamine and derivatives as predictors of gunpowder age by means of HPLC and statistical models. Talanta p. 214-220.
- Chovancova M, Očko P, Pechova A, Lopuch J (2006) Lifetime prediction of propellants according to NATO standards. Problems of Armament Technology 35(98): 7-14.
- Andres BVH, Nitrochemie Bericht Nr (2003) p. 2858.
- Jelisavac L (2010) Life-Time Prediction of Double-Base Propellants in Accordance with Serbian and NATO Standards. Military Technical Institute (VTI) pp. 12-17.
- Bobić N, Simonović R, Drmanić S, Milić S, Nikolić J (2017) The influence of migration processes in gunpowder charge on the quality of mortar ammunition. Hem ind pp. 231-240.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...